This is an Application Brief and does not contain a detailed Experimental section.
Sample loss caused by nonspecific analyte/surface adsorption presents a challenge in the acquisition of accurate and reproducible data. This problem is further compounded by the physicochemical properties of the analyte itself which can exhibit hydrophobic and/or ionic properties that can enhance or inhibit adsorption phenomena. Sample vials represent a surface often overlooked during method optimization. Sample vial surfaces can vary in composition and purity of material which can impact adsorption activity as well as sample stability in terms of denaturing or aggregation events. This is particularly true for charge bearing species such as sialylated or phosphorylated glycans that can exhibit high adsorption activity and are prone to precipitate in sample matrices. This work demonstrates how QuanRecovery Sample Vials with MaxPeak High Performance Surfaces (HPS) Technology can be used to increase recovery and stability of challenging analytes in the analysis of released glycans using HILIC-FLR/MS.
Increased sample recovery leading to improve HILIC-FLR/MS assay accuracy for released glycans
Glycosylation is often designated as a critical quality attribute (CQA) of biotherapeutics due to its impact on drug safety and efficacy. Therefore, accurate characterization and monitoring of glycosylation is required throughout the development process to ensure product quality and consistency. The glycoprofile, including identity and relative abundance of glycans, can be obtained via the release, and labeling of glycans followed by hydrophilic interaction chromatography (HILIC) coupled to fluorescence (FLR) and/or MS detection. However, HILIC-FLR/MS analysis can suffer from low or inconsistent peak recovery, which is partly due to the nonspecific adsorption of analytes to surfaces encountered in the preparation and separation of samples at the bench and on the LC system. While efforts are made to minimize the nonspecific adsorption to LC fluidic paths and columns, as demonstrated by the recent success of ACQUITY Premier LC and columns featuring MaxPeak HPS Technology, the LC sample vials is also a source of sample loss that might often be overlooked in method development. These losses, which can increase over time as samples are queued up for analysis require solutions that can easily deployed in the lab and are compatible with existing methods. In this application brief, we demonstrate the use of QuanRecovery Sample Vials with MaxPeak HPS Technology to mitigate the sample loss and improve the data quality for released glycan assay.
Prior to LC injections, the glycan samples are typically stored in the vials within the autosampler, therefore the in-solution stability of released glycans is critical in the acquisition of consistent and reproducible data. Here we report an integrated solution to maximize the glycan recovery in HILIC-FLR/MS analysis by minimizing nonspecific analyte/surface interaction using the QuanRecovery Sample Vials in addition to the BioAccord LC-MS System and the ACQUITY Premier BEH Amide Glycan Column. Using the Glycan Performance Test Standard (P/N: 186007983) as an example, the consistency of FLR responses over repetitive injections was evaluated as a measure of recovery and in-solution stability of glycans. The sample was reconstituted in the original container (polypropylene vial), followed immediately by transferring half of the reconstituted sample to a QuanRecovery Sample Vial. Figure 1A showed the FLR responses of a sialylated glycan, FA2BG2S2 (F=Fucose, A = Antennary, B=bisecting, G=Galactose, S=Sialic acid), over 15 consecutive injections. Compared to using the original polypropylene vial, nearly 2-fold higher and more consistent FLR responses were obtained when using the QuanRecovery Sample Vial, suggesting MaxPeak HPS Technology enhances the recovery and in-solution stability of sialylated glycans. The consistency of glycan recovery was also evaluated for a protein bearing phosphoglycans, Cathepsin D, where the mannose-6-phosphate (M6P) plays an important role in the treatment of lysosomal storage diseases. It was reported that the phosphate glycans are challenging to recover from HILIC analysis due to their low abundance nature and adsorption to LC system. Recently, it was demonstrated the ACQUITY Premier System coupled with the ACQUITY Premier BEH Amide Column improved the recovery of the Mannose-7-phosphate glycans.1 Here we took a step further, utilizing the QuanRecovery Vial to improve the detector (FLR and MS) response consistency. As shown in Figure 1B, consistent MS response of M6P was obtained over 15 injections, demonstrating QuanRecovery Vials, as part of an integrated solution, offers analysts the means to increase recovery of challenging glycans and acquire more consistent data in the analysis of glycans.
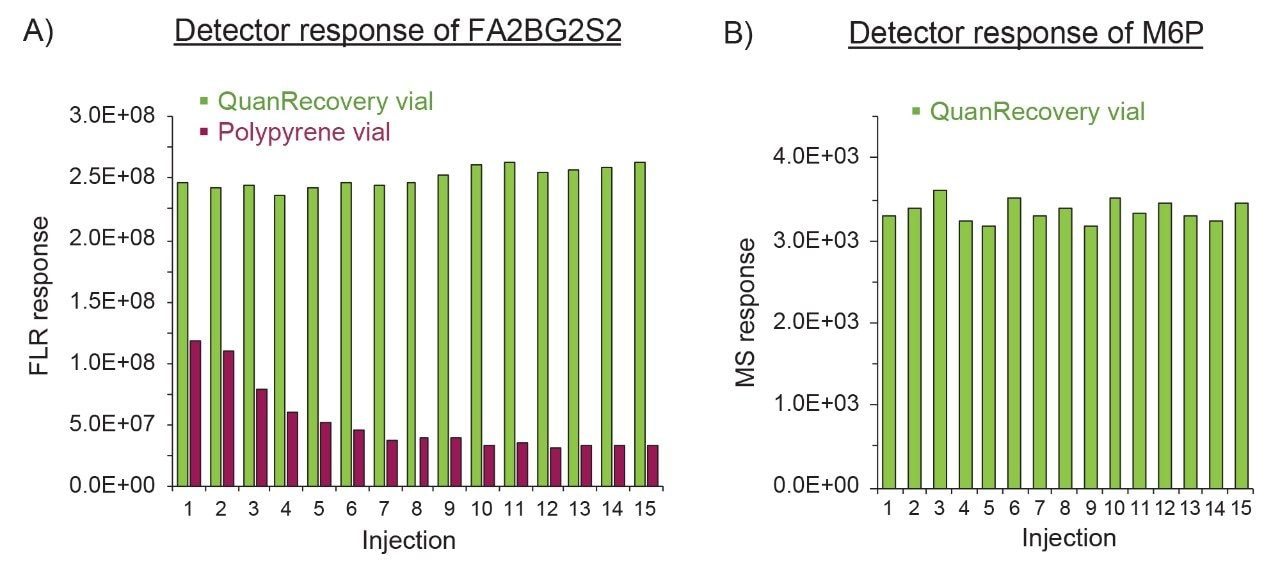
This application brief demonstrates the benefit of using QuanRecovery Sample Vials with MaxPeak High Performance Surfaces (HPS) Technology for HILIC-FLR/MS analysis of released glycans. Increased consistency and recovery of released glycans were observed for the Glycan Performance Test Standard and phosphoglycans, which may be attributed to the reduction of nonspecific analyte/surface interaction encountered with commonly used polypropylene vials. In combination with the ACQUITY Premier Solution, more accurate and consistent HILIC-FLR/MS analysis of released glycans can be achieved, therefore enabling more robust methods that can be readily deployed in the development and manufacturing of biotherapeutics.
720007308, July 2021