
This application note illustrates specific, targeted method for the determination of glyphosate, glufosinate and its metabolites in soybean extracts.
*Please note: This Application Note was developed on a Torus DEA Column, improved performance can be achieved using the Waters Anionic Polar Pesticide Column coupled with our most recent Application Notes please contact Waters Chemistry Technical Services with any questions www.waters.com/contact.
Specific, targeted method for determination of glyphosate, glufosinate and its metabolites in soybean extracts suitable for monitoring compliance with MRLs/tolerances.
Glyphosate (N-(phosphonomethyl)glycine) and glufosinate (2-amino-4-(hydroxymethylphosphinyl)butanoic acid) have multiple uses; as non-selective herbicides widely used in agriculture and municipal applications, as foliar sprays for desiccation of certain crops before harvest, and as selective herbicides for use on genetically modified, herbicidetolerant crops. In genetically modified plants, the metabolic patterns for glyphosate and glufosinate are driven by the modifications introduced into the genome of the plant resulting in additional significant metabolites. For checking compliance with the US tolerance of 2 mg/kg for glufosinate in soybean,1 one needs to measure the sum of glufosinate and its metabolites, 2-(acetylamino)-4-(hydroxymethyl phosphinyl)butanoic acid (N-acetyl glyphosate [NAG]), and 3-(hydroxymethylphosphinyl)propanoic acid (MPPA), expressed as glufosinate equivalents. Compliance with the US tolerance of 30 mg/kg for glyphosate in soybean2 is determined by measuring glyphosate and its metabolite N-acetyl-glyphosate; calculated as the stoichiometric equivalent of glyphosate.
Glyphosate, glufosinate and their metabolites are highly polar ionic compounds and remain a considerable challenge for the analytical scientist. Numerous analytical procedures have been published in the literature for the determination of glyphosate with one of the most common approaches being derivatization fluorenylmethyloxycarbonyl chloride (FMOC-Cl). Although such methods are well established, the N-acetyl metabolites of glyphosate and glufosinate cannot be derivatized using FMOC, and such approaches are not suitable for checking compliance in situations where the residue definition includes these metabolites.
Previously, we have reported on the performance of the Torus DEA Column used as a UPLC column for the determination of polar anionic pesticides and their metabolites in spinach and wheat using the QuPPe method.3,4 The Torus DEA is a UPLC column that provides HILIC and WAX interactions, which has been shown to offer sufficient retention of these highly polar and ionic compounds while providing excellent retention time stability, selectivity, and peak shape. In this application note, we report the performance data from an assessment of a similar LC-MS/MS method, but using an alternative published extraction method,5 for the determination of glyphosate and glufosinate in soybean extracts. This extraction method uses water containing EDTA and acetic acid to precipitate protein and extract the analytes into solution. The SPE cartridge is used in a “pass-through” mode to retain suspended particulates and non-polar co-extractives.
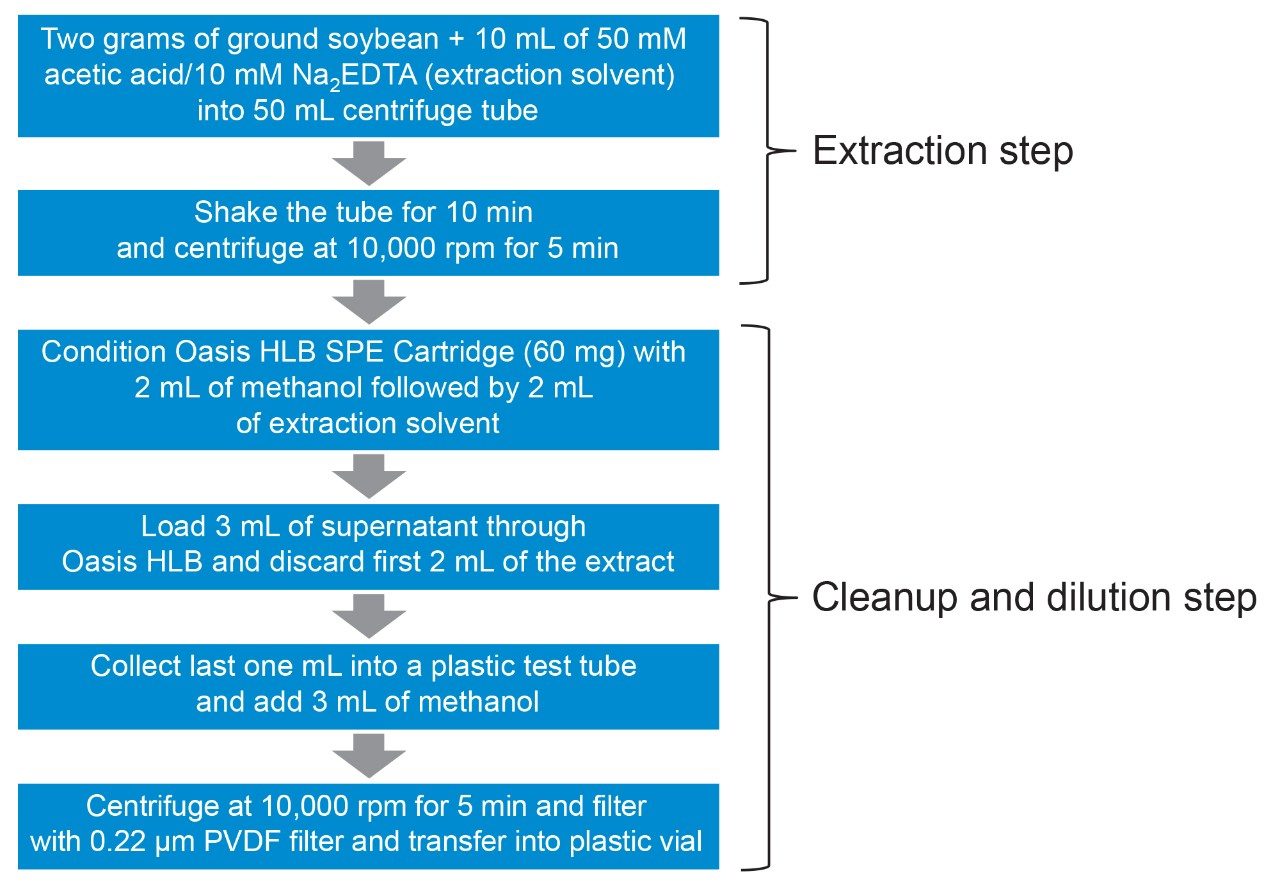
A sample of soybean, representative of a commodity with high fat and/or protein content and low water content, was purchased from a retail outlet. Soybean was homogenized in a blender and test portions extracted using a previously published method,5 summarized in Figure 1.
The performance of the UPLC-MS/MS step of the method was assessed using the widely accepted SANTE guidelines.6 Matrix-matched standards were prepared over the range 2.5 to 40 mg/kg for glyphosate and its metabolites, and 0.25 to 4 mg/kg for glufosinate and its metabolites. All extracts, standards, and blanks were diluted 1:3 with methanol before UPLC-MS/MS. The standards were used to create bracketed calibration graphs for the replicate analyses of a matrixmatched standard at 5 and 0.5 mg/kg for residue definition of glyphosate and glufosinate, respectively.
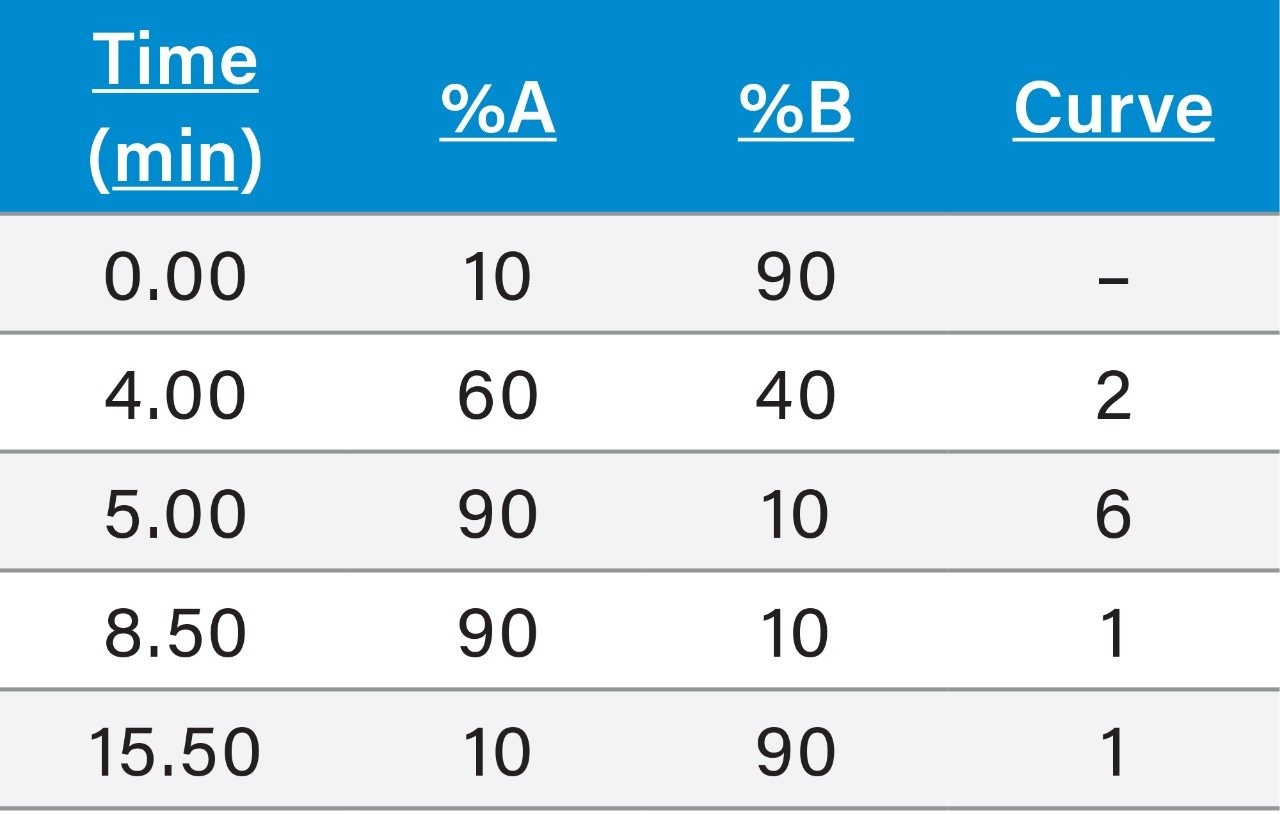
Before use, the LC system and column required simple cleaning and conditioning steps to remove metal ions that have been shown to interact with polar pesticides and cause poor peak shapes. Details can be found in the start-up guide7 (720006156EN).
|
UPLC system: |
ACQUITY UPLC H-Class Bio with FTN Sample Manager |
|
Column: |
Torus DEA 1.7 μm, 2.1 mm × 100 mm (p/n 186007616) |
|
Mobile phase A: |
50 mM ammonium formate + 0.9% formic acid |
|
Mobile phase B: |
acetonitrile + 0.9% formic acid |
|
Flow rate: |
0.5 mL/min |
|
Injection volume: |
10 μL |
|
Weak wash solvent: |
90:10 acetonitrile:water |
|
Strong wash solvent: |
10:90 acetonitrile:water |
|
Column temp.: |
50 °C |
|
Sample temp.: |
10 °C |
|
Run time: |
16 min |
|
MS instrument: |
Xevo TQ-XS |
|
Ionization: |
ESI- |
|
Capillary voltage: |
2.5 kV |
|
Ion counting threshold: |
250 |
|
Desolvation temp.: |
600 °C |
|
Desolvation gas flow: |
1000 L/Hr |
|
Source temp.: |
150 °C |
|
Cone gas flow: |
300 L/Hr |
|
Collision gas flow: |
0.14 mL/min |
|
Nebulizer gas pressure: |
7 Bar |
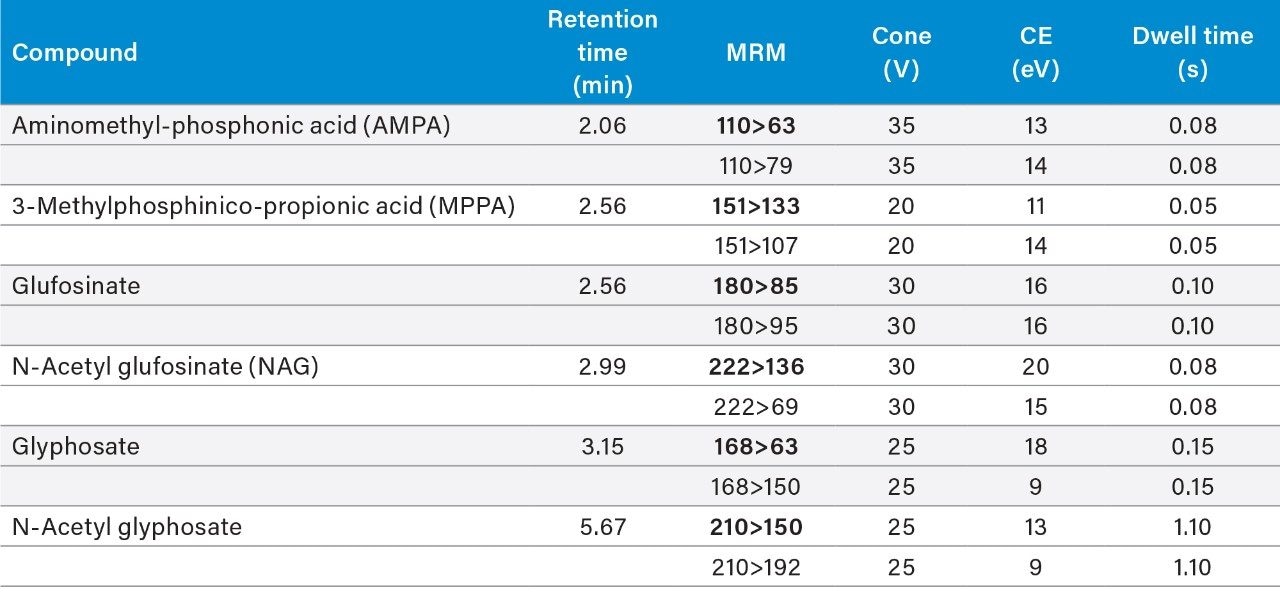
The data was acquired using MassLynx v4.2 Software and processed using TargetLynx XS Application Manager. The selection of MRM transitions and optimization of critical parameters was performed by infusion of individual solutions of all the analytes and evaluation of the data with Intellistart to automatically create acquisition and processing methods.
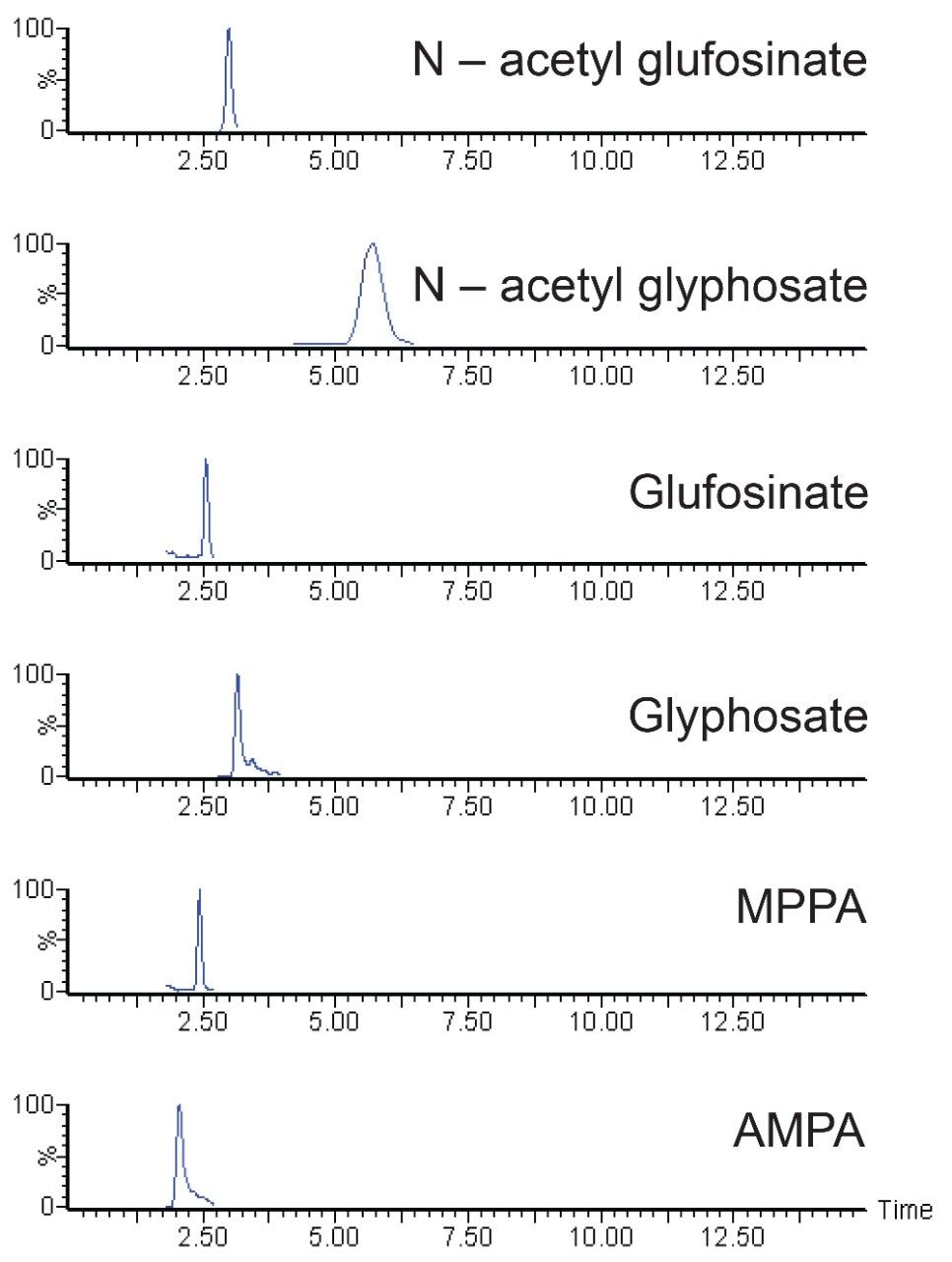
Excellent sensitivity and selectivity was demonstrated from the analysis of matrixmatched standards. Figure 2 shows the chromatography and response for the compounds making up the residue definition for glufosinate and glyphosate at 0.5 and 5 mg/kg in soybean, respectively.
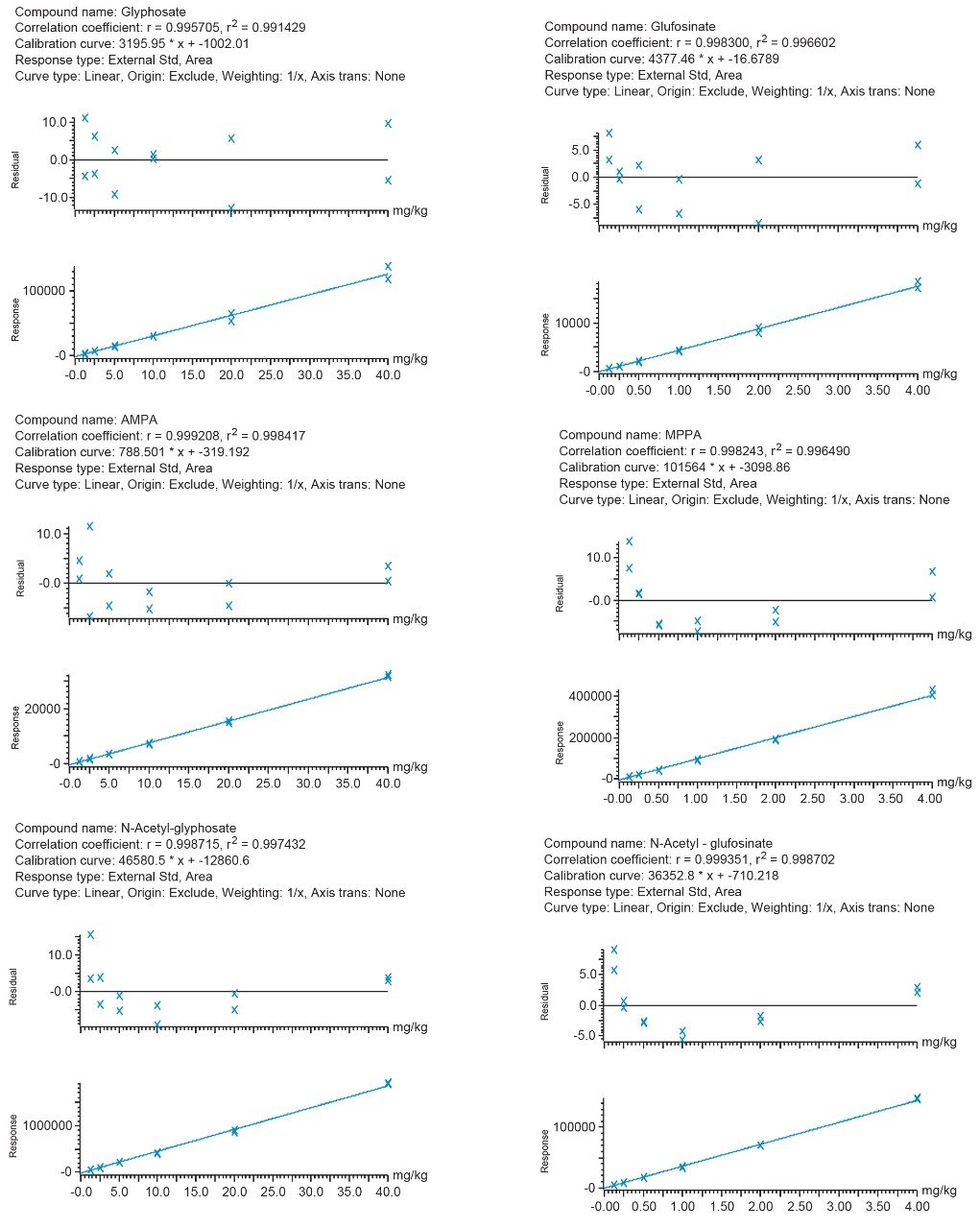
Calibration was assessed for the pesticides of interest by use of bracketed calibration over a suitable concentration range, as shown in Figure 3. The coefficients of determination (r2>0.99) and the residuals (<20%) were excellent, demonstrating good repeatability of the measurements, in the absence of labeled standards. Peak shapes remained stable without deterioration through the run. Replicate (n=9) injections of soybean spiked at 0.5 and 5 mg/kg showed good precision, with RSDs ≤6%, for glufosinate (and its metabolites) and glyphosate (and its metabolites), respectively.
Ion ratios and retention times agreed well with reference values and were within the required tolerances (±30% and ±0.1 minutes, respectively).
The LC-MS/MS method performance has been determined to be suitable for monitoring tolerances for the compounds of interest in soybean. The scope of the analysis includes all components that make up the residue definition.
The use of the Torus DEA Column provides excellent chromatographic performance for glyphosate, glufosinate and metabolites, even for the analysis of a complex and difficult matrix such as soybean. When coupled with the high sensitivity of the Xevo TQ-XS Mass Spectrometer, these challenging compounds can be determined in a single analysis, after dilution, without relying on derivatization or specialized equipment. When used in combination with established extraction protocols, it is suitable for checking MRL/tolerance compliance. This application note shows that this Torus DEA method is suitable for analysis of aqueous extracts in addition to those from QuPPe.
Scientists must fully validate the method on their commodities of interest, in their own laboratories, to demonstrate that, when coupled with their extraction protocols, it is fit for purpose.
720006404, February 2019