
This application note describes a fast, simple, and reliable method for the QC and profiling of flavonoids in citrus species using the ACQUITY UPLC H-Class System and the ACQUITY QDa Detector.
Flavonoids belong to a group of naturally occurring polyphenolic compounds found in many sources including citrus fruits.1 There are two important sub groups: flavanones and flavones. Flavones are also known as polymethoxylated flavones (PMF). The most common flavanone is hesperetin from oranges; and the most common flavones are tangeretin and nobiletin in orange and tangerine peels.2 The analysis of flavonoids in citrus species is often used for quality control and product authenticity testing because of their remarkable taste properties and variation of flavonoid profiles in the different species and varieties of citrus.3,4 Figure 1 shows the chemical structures of the flavonoids used in this study.

The most common technique for flavonoids analysis is reversed-phase liquid chromatography with UV/Visible absorbance detection (UV/Vis) or Photodiode Array detection (PDA). PDA is preferred over UV/Vis since it can provide spectral data and facilitate peak purity checks. The challenge with PDA analysis is that flavonoids consist of dozens of structurally similar compounds, typically differing only in the degree of ring substitution, the type of substitution, and the type and degree of glycosylation. PDA detection lacks the selectivity to distinguish the subtle structural difference in flavonoids. Mass spectrometry (MS) can provide better selectivity (except isobaric compounds) and better sensitivity for flavonoids analysis than PDA detection. However, the cost and the ease of use of MS instruments can be an obstacle towards the adoption of MS for flavonoids analysis, especially for QC labs in manufacturing plants.
Waters ACQUITY UPLC H-Class System with the ACQUITY QDa Mass Detector offers the best balance of selectivity, usability, and affordability for flavonoids analysis. The ACQUITY QDa is designed to provide mass spectral data with minimal tuning at an affordable cost. It not only extends the sample detection coverage of optical detection to compounds with no UV or fluorescence chromophore, but it also improves the selectivity of compounds of similar structures, which is very useful in flavonoids analysis.
|
LC system: |
ACQUITY UPLC H-Class |
|
Detection: |
ACQUITY PDA |
|
Column: |
ACQUITY UPLC HSS T3 1.8 μm, 2.1 x 100 mm, (p/n 186003539) |
|
Column temp.: |
35 °C |
|
Sample temp.: |
4 °C |
|
Injection volume: |
2.0 μL |
|
Flow rate: |
0.6 mL/min |
|
Mobile phase A: |
0.1% Formic acid in deionized water |
|
Mobile phase B: |
0.1% Formic acid in acetonitrile |
|
Run time: |
13 min |
|
Time (min) |
A% |
B% |
Curve |
|---|---|---|---|
|
Initial |
98 |
2 |
6 |
|
5.5 |
27 |
73 |
6 |
|
6.0 |
0 |
100 |
6 |
|
10.0 |
0 |
100 |
6 |
|
10.5 |
98 |
2 |
6 |
|
13.0 |
98 |
2 |
6 |
|
MS system: |
ACQUITY QDa |
|
Ionization mode: |
ESI+ |
|
Acquisition: |
SIR |
|
Cone voltage: |
5 V |
|
Probe: |
Default (600 °C) |
|
Capillary: |
Default (0.8 kV) |
|
Sampling freq.: |
Default (5 Hz) |
Data were processed using MassLynx MS Software
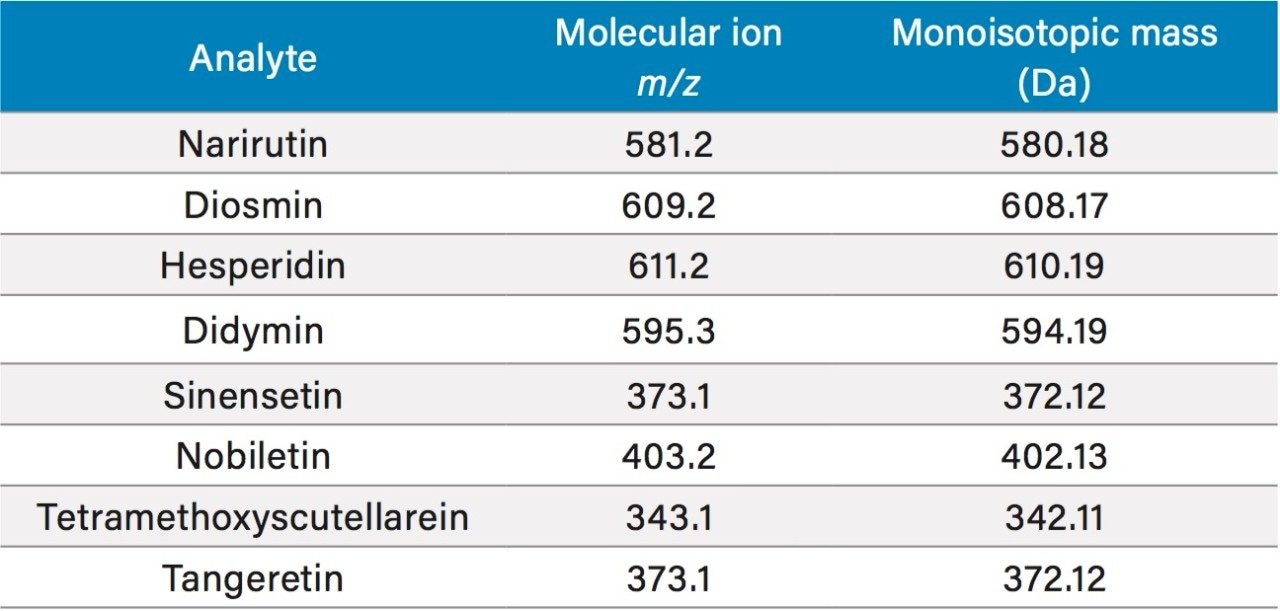
The detection of flavonoids was achieved by selective ion recording (SIR) of the molecular ions of the flavonoids of interest. Table 1 shows the monoisotopic masses of the flavonoids and their molecular ion mass-to-charge ratios (m/z). Most of them have different m/z values, except for only one pair of compounds, the sinensetin and tangeretin, which both have the same m/z value. The sinensetin and tangeretin can be easily separated chromatographically, so they will not interfere with each other. Figure 2 shows the chromatograms from UV (325 nm) and from ACQUITY QDa detection for a juice sample. As can be seen in Figure 2, sinensetin (peak E) and tangeretin (peak H) elute at different times. The nobiletin and the tetramethoxyscutellarein peaks partially overlap each other. The quantitation of these peaks is a challenge using UV/Vis or PDA detection, but it is not an issue with the ACQUITY QDa Detector, since these two analytes can be selectively monitored at m/z 403.2 and 343.1, respectively.
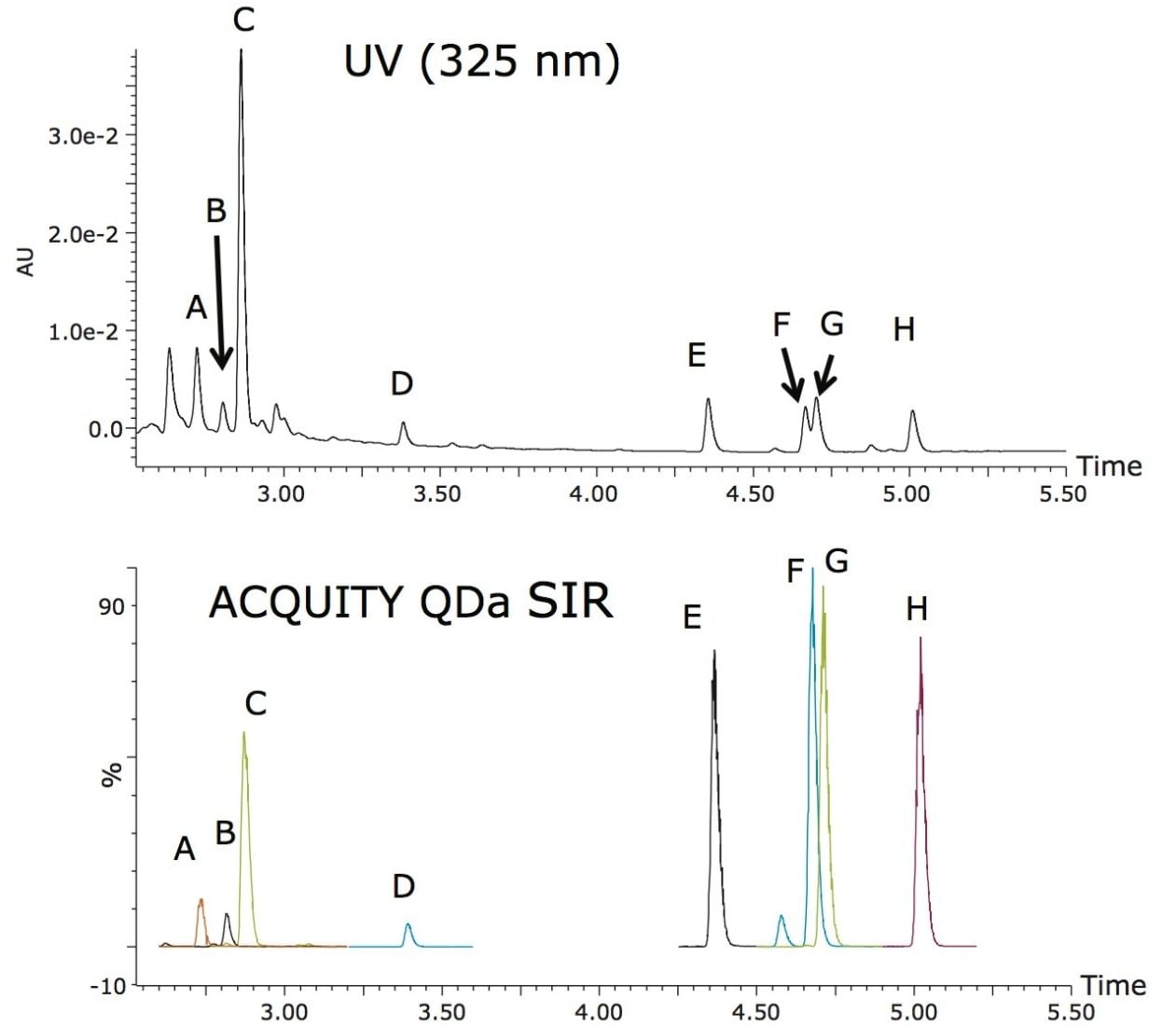
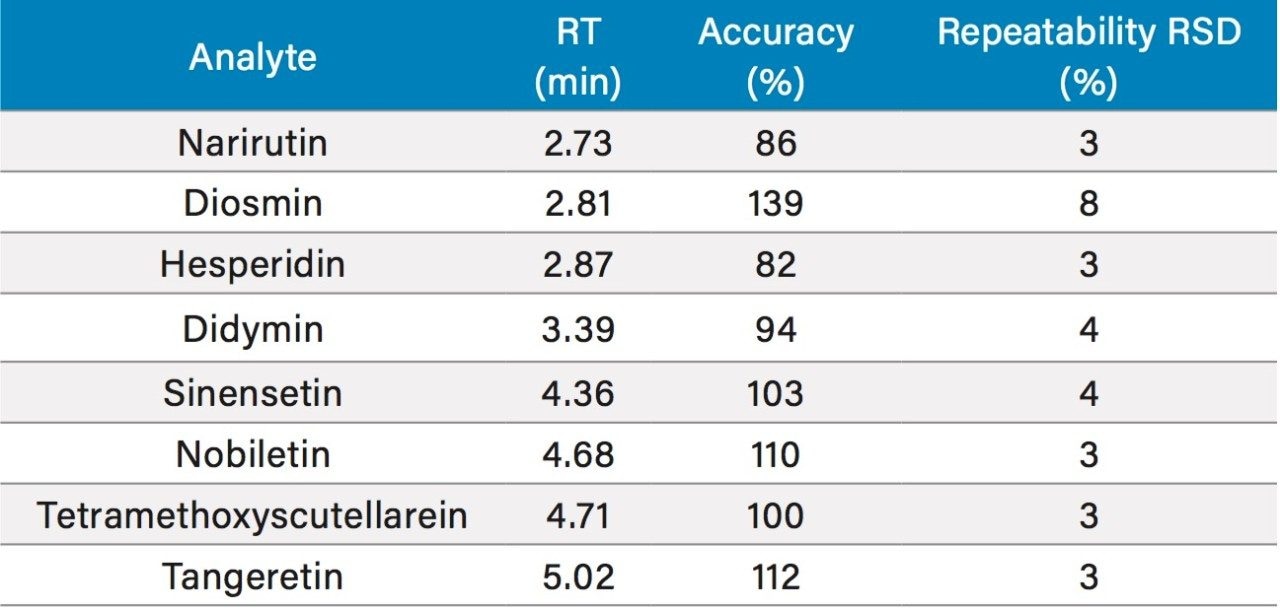
The selectivity of the ACQUITY QDa Mass Detector allows for simplified chromatographic method development work. It is acceptable to have a chromatographic method without baseline separation, as long as the compounds can be selectively detected. With UV/Vis or other optical detectors, it is required to obtain chromatographic baseline resolution for all analytes, which can be a challenge for structurally similar compounds, and it often results in longer run times. The total injection cycle time of the method presented here, including column equilibration, is 13 minutes, which is at least four times faster than existing methods. Table 2 is a summary of analysis results for a fruit juice. The analytical performance is comparable to the existing QC method.
Waters ACQUITY UPLC H-Class System with the ACQUITY QDa Mass Detector provides increased selectivity for the detection of flavonoids in citrus juices as compared to optical detectors that are typically used. The improved detection and selectivity simplify analytical method development, allowing for faster and easier chromatographic methods, as well as faster and more efficient sample preparation procedures. These improvements in turn increase lab productivity and sample throughput. In addition, the ease of use and affordable cost of the ACQUITY QDa Detector make it an essential tool for routine analyses.
720004985, November 2016