바이러스 벡터 기반 유전자 치료제는 200,000가 넘는 원자를 포함하는 단백질 및 핵산 성분으로 구성된 고분자 의약품 제형입니다. Waters의 액체 크로마토그래피(LC)는 광학 검출(UV, MALS) 및 질량 분석(MS)과 결합되어 이러한 이질적인 구조와 특성을 구성 성분 수준 및 인택트(intact) 수준 모두에서 정확하게 특성화 및 정량할 수 있는 분석 수단을 제공하며, 이에 따라 견고한 바이러스 벡터 솔루션을 지원합니다. 일반적인 측정 항목에는 바이러스 벡터 솔루션 워크플로에서의 바이러스 역가, 응집체/불순물 분석, 바이러스 단백질 비율, 펩타이드 매핑 및 변형 분석, 캡시드화 효율성 및 게놈 완전성 평가가 포함됩니다.
웹세미나: 하전 검출 질량 분석기(CDMS)를 통한 혁신과 산업의 연결
Intact mass 분석, 서열 확인, 공정 모니터링 등을 위한 앱과 함께 생체 분자 및 바이러스 벡터 솔루션의 분석을 위한 waters_connect를 사용하면 시료 분석에서 의사 결정에 이르기까지의 과정을 빠르게 추진할 수 있습니다.
실험실에 Empower 크로마토그래피 데이터 시스템(CDS)을 갖춤으로써, 바이러스 벡터 분석을 위한 수집, 처리 및 보고를 포함한 고급 실험실 데이터 관리 기능을 확보하세요.
기기 제어, 데이터 수집, 분석 및 보고는 모두 Viral Vector Analysis 모듈이 포함된 ASTRA 소프트웨어를 통해 제공됩니다. Waters는 AAV 정량을 위해 특별히 구축된 플랫폼 SEC-MALS 분석법을 제공하며, 이는 모든 제품과 혈청형에 맞게 쉽게 사용자 정의할 수 있습니다.
분석 시간을 단축하고 시료의 소모를 줄이는 Waters의 고효율 GTxResolve Premier SEC 컬럼을 통해, 응집 및 크기 변형 분석을 포함한 여러 속성을 측정할 수 있습니다.
최적화된 염 그래디언트와 비다공성, 고효율의 고정상(예:Protein-Pak Hi Res Q)이 결합되어 empty/full 측정을 위한 QC에 적합한 기술을 사용하는 Waters AEX 크로마토그래피로 회수율을 향상시킵니다.
탁월한 LC 및 MS 성능을 위해 DFA 이온쌍을 갖춘 MaxPeak Premier BEH C4 컬럼을 사용하여 바이러스 단백질의 상대적 존재비를 식별 및 측정합니다.
바이러스 벡터, 캡시드 단백질, 핵산의 배치 간 재현성을 높일 수 있도록 설계된 Amide 고정상. 산화 및 인산화 변이체는 최적의 HILIC 분리를 적용하여 비변형 형태로부터 쉽게 분리할 수 있습니다.
펩타이드 맵핑과 함께 이러한 응용 분야에 대해 고분리능을 제공하는 Waters CSH™ 컬럼을 사용하여 바이러스 단백질의 시퀀스, 번역 후 변형(PTM) 또는 분해를 확인할 수 있습니다.
Waters 글로벌 서비스를 통해 피크 시스템 성능을 유지하고, 가동 중지 시간을 줄이며, 응용 과제를 해결하고 엄격한 규제 준수 요구 사항을 준수함으로써 실험실의 생선성과 성공 가능성을 개선하세요.
노후 장비 업그레이드, 맞춤형 지원 이용, 월간 결제 요금에 서비스 번들화 등 Waters Capital의 결제 옵션을 이용하여 리소스를 충분히 활용하고 위험을 감소하세요.
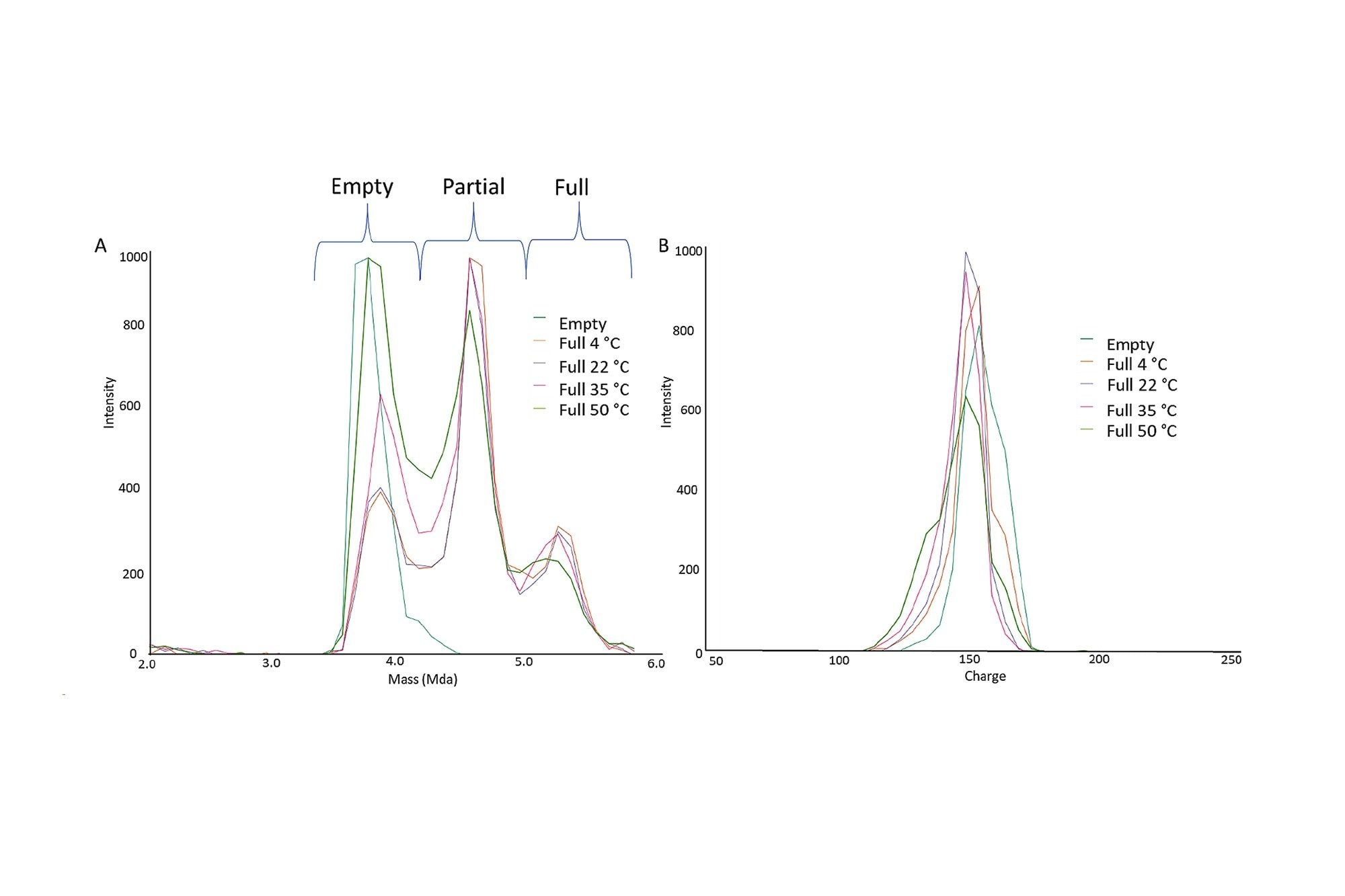
a) 2.5-6 MDa 질량 범위로 확대하여 표시한 AAV8의 오버레이 질량 스펙트럼. 100% Empty/Full 캡시드를 4, 22, 35 및 50°C에서 30분 동안 처리한 결과입니다. b) 2.5-6 MDa 질량 범위로 확대하여 표시한 AAV8의 전하 스펙트럼. 100% Empty/Full 캡시드를 4, 22, 35 및 50°C에서 30분 동안 처리한 결과입니다.
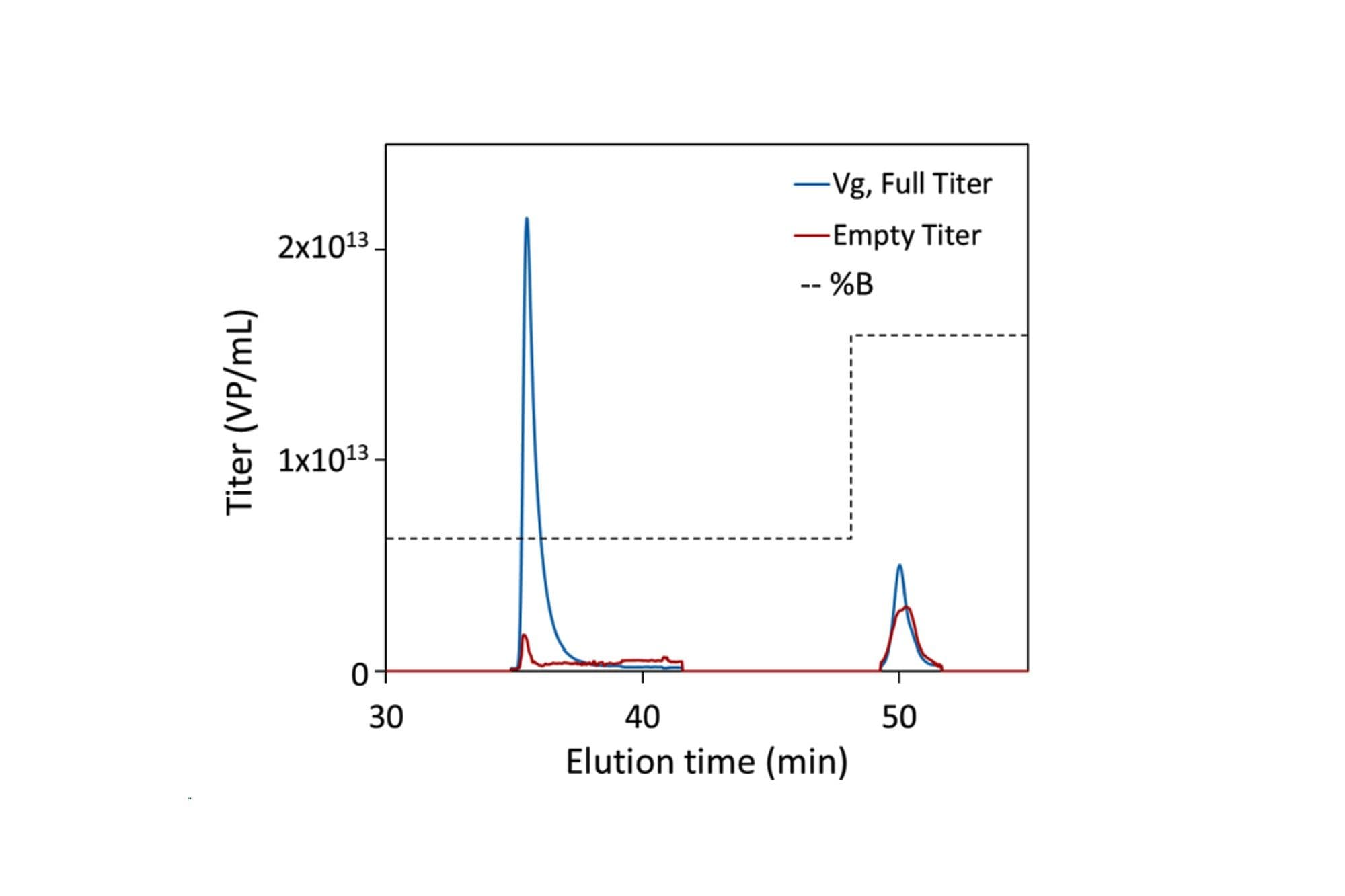
최종 선형 기울기에서 용리(34~48분) 및 스트립(>48분) 중 RT-MALS에 의해 결정된 Full(파란색) 및 Empty(빨간색) 역가. 완충액의 이온 강도는 검은색 점선으로 표시됩니다.
스테인리스 스틸 하드웨어(4.6 x 150mm, 5µm 입자, 빨간색 트레이스) 및 MaxPeak HPS 하드웨어(XBridge Premier GTx BEH SEC 450Å 2.5µm 4.6 x 150mm 컬럼, 검은색 트레이스)를 통해 얻은 AAV2 크로마토그램의 확대 보기. 표준 이온 강도 완충액(10mM 인산염 pH 7.4 + 200mM KCl)이 포함된 이동상을 사용하여 분리를 수행했습니다
VP 단백질의 상대적 정량은 (A) UV 및 (B) 형광(FLR)을 포함한 광학 검출을 통해 측정했습니다. 피크의 주석은 검출된 성분의 할당 및 계산된 상대 존재비를 표시합니다. FLR 검출 사용 시, VP3의 S/N은 UV 검출에 비해 거의 5배 높으며, 이때 질량 부하는 10배에 달하므로 결과적으로 감도가 약 50배 향상됨을 알 수 있습니다.
