Supporting Pharmaceutical Synthetic Process R&D Using LC-MS
This is an Application Brief and does not contain a detailed Experimental section.
Abstract
This application brief demonstrates enhancements to UPLC-MS technology and its use with open access software to improve synthetic process monitoring for process R&D chemists.
Benefits
Introduction
Once a chemical hit is found through a library screening process and is verified, investigation of the compounds’ synthetic route takes place. This step involves an iterative process of different synthetic approaches to generate the identified compound efficiently and safely.
Because these reactions may take a long time, chemists need to know as soon as possible if their syntheses are proceeding as desired. This means utilizing measurement capabilities that require minimal sample preparation and provide a fast response giving low detection limits.
High throughput approaches can provide important time savings in the optimization of process parameters and help identify false positives. Open access liquid chromatography coupled to mass spectrometry (LC-MS) is replacing thin layer chromatography (TLC) as a reaction monitoring tool. Sample preparation of reaction mixtures can be as minimal as filtering and dilution before injecting into the LC-MS system. This allows fast turnaround of results to allow the chemist to advance to the next step or to eliminate the process.
Thus, sample throughput is a critical issue for process R&D chemists. Ultra-Performance Liquid Chromatography (UPLC) leverages sub-2-µm LC particle technology to generate high efficiency and faster separations.
Open access software offers the power of chromatography and mass spectrometry to chemists who are not analytical instrumentation specialists. It allows them to quickly and easily know what they’ve made and allows the experts to work on the difficult analytical problems.
An open access UPLC-MS system was investigated for process R&D support. This application brief describes some of the enhancements to LC and LC-MS technologies that have generated useful tools to improve the throughput and accuracy of pharmaceutical synthesis assays.
Experimental
LC conditions
|
LC system: |
ACQUITY UPLC with SQ Detector 2 |
|
Column: |
ACQUITY UPLC BEH, 2.1 x 30 mm, 1.7 μm, p/n: 186002349 |
|
Mobile phase A: |
0.1% Formic acid in water |
|
Mobile phase B: |
0.1% Formic acid in acetonitrile |
|
Gradient: |
5% to 95% B over 2 minutes |
|
Flow rate: |
800 μL/min |
|
Column temp.: |
50 °C |
|
Sample temp.: |
8 °C |
|
Injection volume: |
2.0 μL |
MS conditions
|
MS system: |
SQ Detector 2 |
|
Ionization mode: |
ESI+/ESI- |
|
Acquisition range: |
150 to 600 m/z |
|
Capillary voltage: |
3.0 KV |
|
Cone voltage: |
20 V |
|
Desolvation temp.: |
450 °C |
|
Desolvation gas: |
900 L/Hr |
|
Source temp.: |
150 °C |
Results and Discussion
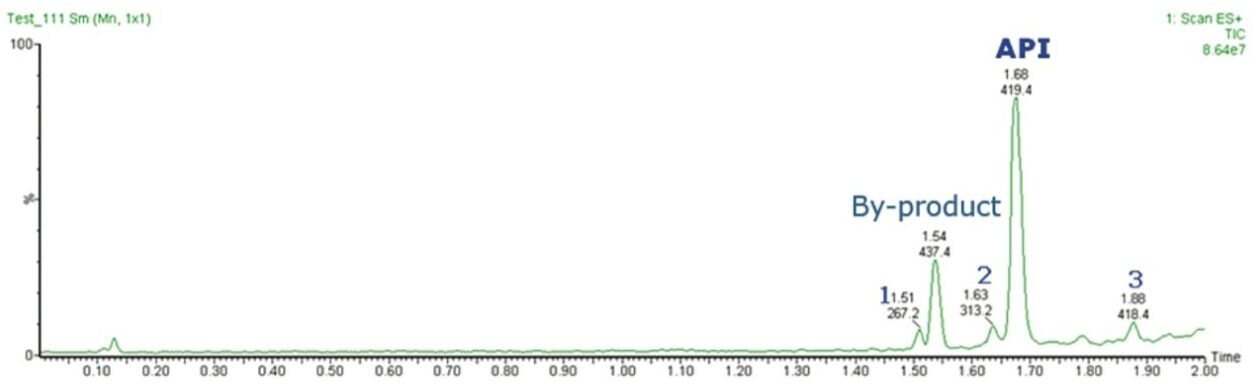
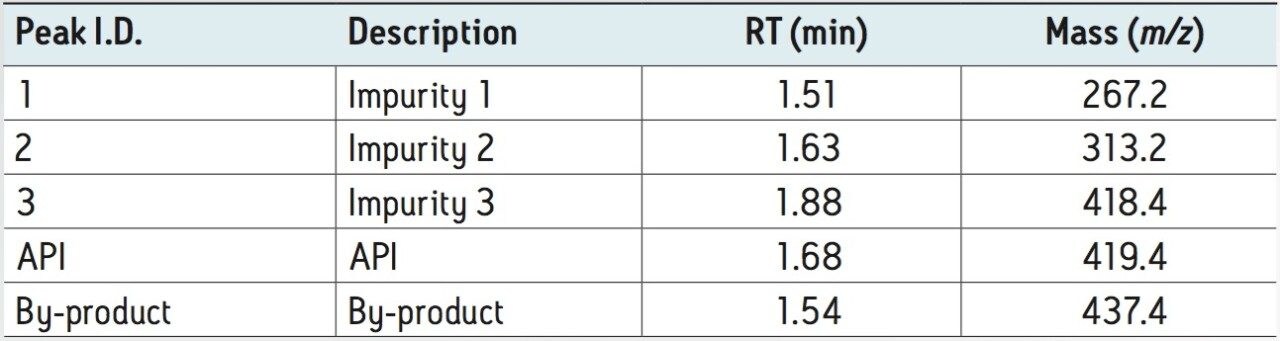
A representative sample from a synthetic chemistry laboratory was analyzed a using gradient of 2 minutes. Chromatographic separations were carried out using an ACQUITY UPLC System coupled to an SQ Detector 2 single quadrupole mass spectrometer, using OpenLynx Application Manager for MassLynx Software.
By using a walk-up UPLC-MS system, chemists were able to quickly and easily determine the successful completion of their reactions with mass confirmation, noting the API synthesis as well as seeing the formation of any side products or other impurities. As a consequence chemists can spend less time setting up and analyzing the samples, get mass confirmation quicker than previously, and therefore increase their reaction throughput.
Conclusion
Synthetic chemists frequently need to confirm the identity of synthesized compounds and confirm the presence of any impurities quickly and easily using very small quantities of material in the quickest possible time. The ability to do this using an open access LC-MS system utilizing UPLC with the SQ Detector 2 allows them to make rapid, informed, high quality, data rich decisions on their synthetic processes thereby enhancing their productivity and improving their support of the drug discovery and development process.
Featured Products
720004065, February 2024