
For forensic toxicology use only.
This is an Application Brief and does not contain a detailed Experimental section.
This application note describes the application of Tof-MRM using a Xevo G2-XS QTof with UNIFI Scientific Information System for the analysis of forensically-relevant drugs in urine.
The use of Tof-MRM to confirm the presence, or absence, of 12 drugs in authentic urine using the Xevo G2-XS QTof and UNIFI.
Laboratory testing for illicit drug substances frequently employs a combination of immunoassay-based screening, for common drug classes, followed by confirmatory testing using targeted LC-MS/MS based techniques. Some service providers have successfully replaced this multiple step approach with a single LC-MS/MS procedure targeted for a selection of key analytes.¹ While both of these strategies represent an effective procedure for a limited panel of analytes, the approach does not provide information for a broad range of drug substances. Additionally, these methods are also unlikely to include some of the newer, emerging drug substances.
Previously we have described a time-of-flight (Tof) screening method with the potential to screen for an unlimited number of toxicologically-relevant substances within 15 minutes.2,3 This technique employs Tof-MSE, a non-targeted data acquisition mode which yields a complete dataset from which thousands of substances may be screened. The same mass spectrometer may also be used in targeted mode i.e., multiple reaction monitoring mode (Tof-MRM), providing enhanced sensitivity; this mode allows isolation of a precursor mass using the quadrupole followed by Tof detection of specific fragment ions.⁴
Here we present the analysis of 12 common drugs in urine using Tof-MRM. The technique uses the same well-established chromatographic separation as that used for the Forensic Toxicology Screening Application Solution with UNIFI³ and as such, provides the user with ability to perform screening and confirmation on a single platform.
Authentic drug-free urine was collected from volunteers and pooled. The pooled urine was spiked with a mixture of 12 drug substances to yield a series of samples at the following concentrations: 0.05, 0.1, 0.5, 1, 2.5, 5, 10, 25, 50 ng/mL. A blank urine sample was also prepared. Samples were further diluted 5 five-fold with mobile phase prior to analysis.
Data was acquired using the ACQUITY UPLC I-Class (FTN) together with the Xevo G2-XS QTof in Tof-MRM mode. Two product ions (quantifier and qualifier) were monitored for each of the 12 drugs shown in Table 1. All transitions were acquired with a collision energy ramp from 10 eV to 40 eV, with the exception of norbuprenorphine, for which a constant collision energy of 40 eV was used to monitor of the m/z 101.0961 fragment ion.
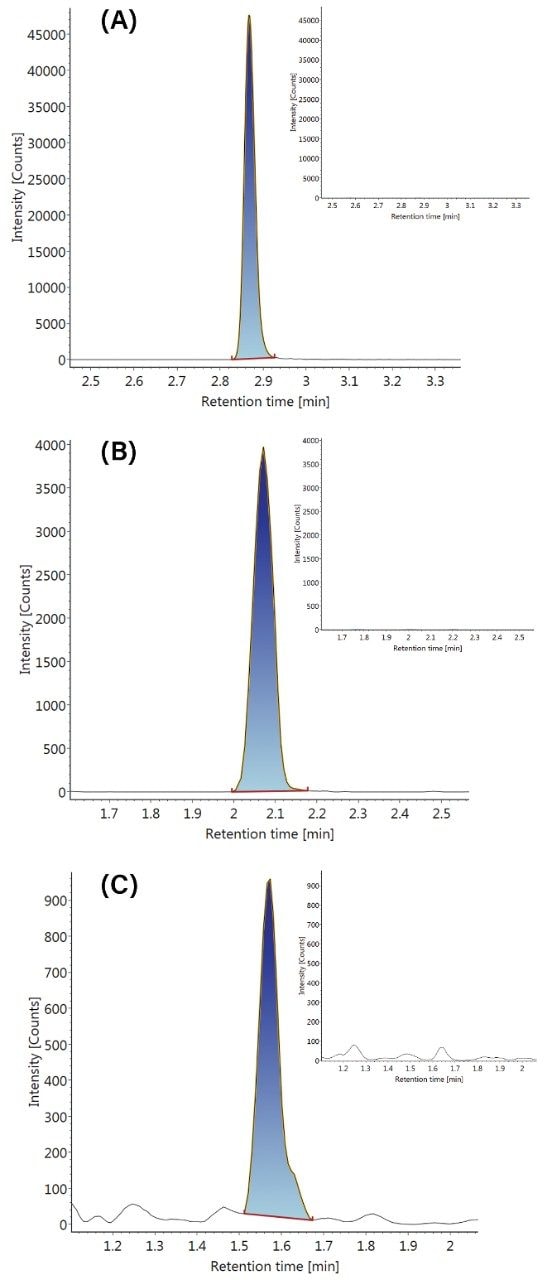
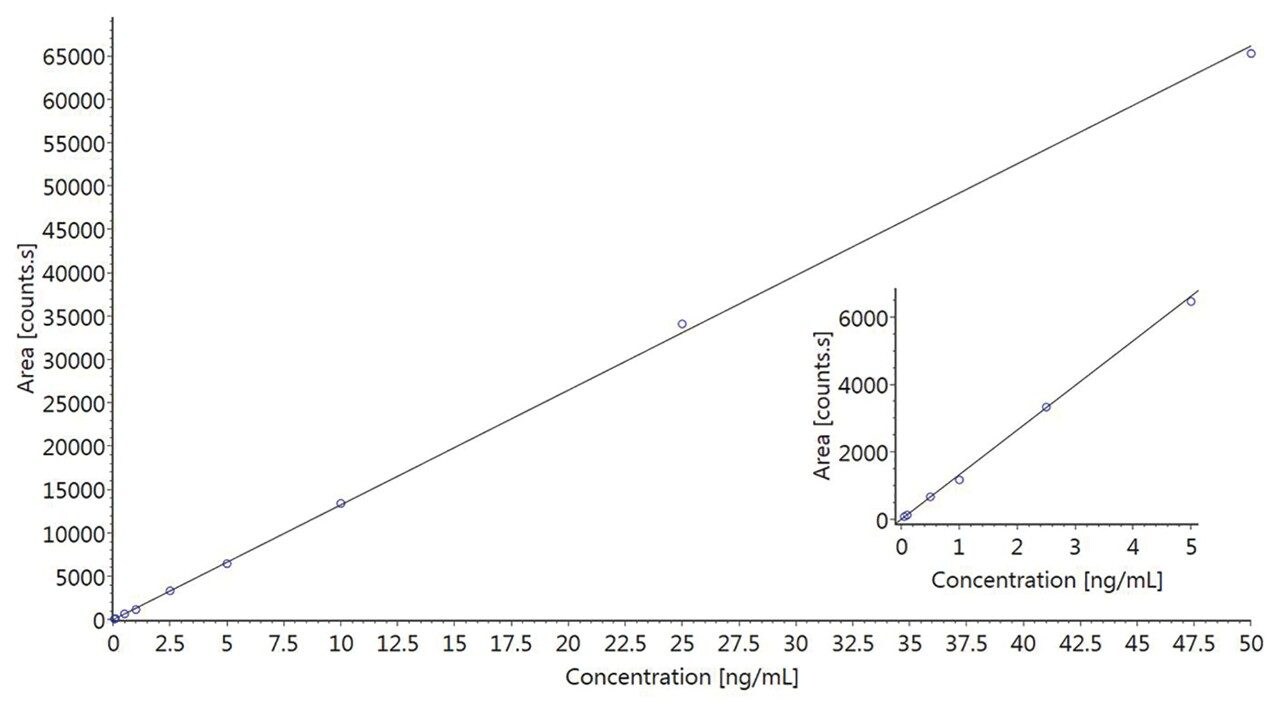
Data were acquired and processed using UNIFI. Processing comprised automatic extraction of the mass chromatogram, for each transition, followed by peak integration. Figure 1 shows representative qualifier chromatograms for three of the substances investigated. The corresponding data for the blank urine sample is included for comparison. Similar results were obtained for the other nine drugs in the study. The standard curve for the quantifier ion of 6-monoacetylmorphine is shown in Figure 2 and demonstrates excellent linearity over the entire concentration range.
Tof-MRM has been successfully used, on the Xevo G2-XS QTof, to analyze a panel of 12 drug substances. The enhanced selectivity of Tof-MRM permitted detection of the analytes in urine spiked at low ng/mL concentrations and prepared by a simple dilution.
Tof-MRM combined with the ability to perform Tof-MSE enables comprehensive non-targeted screening and confirmation on a single platform.
720005943, April 2017