This application note describes a UPLC-MS/MS analysis of all five diastereomers of HBCD and TBBP-A in samples of marine origin. The optimized separation used in this method resulted in a run time of 10 minutes, with the five HBCD diastereomers and TBBP-A analyzed being separated to <10% valley.
Brominated Flame Retardants (BFRs) are chemicals commonly used in many domestic and industrial appliances, equipment and textiles to increase their resistance to fire. The use of BFRs has seen an exponential rise over the last few decades with HBCD and TBBP-A being two of the most common chemicals used in the highest levels.
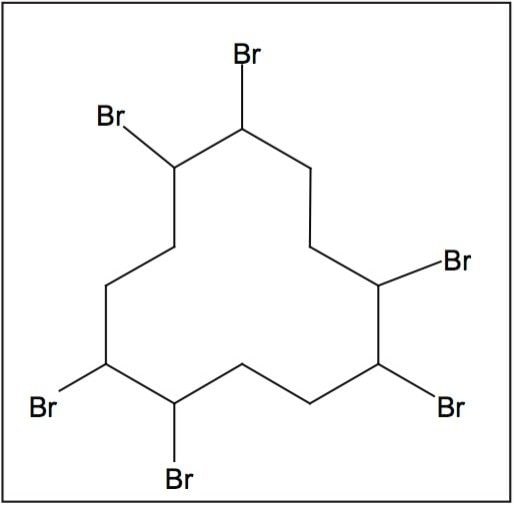
HBCD (Figure 1) is used around the world as a flame retardant in thermal insulation foam for building and construction applications, as well as in upholstery textile coatings, to help prevent deaths and injuries from fire. The HBCD technical product is composed of a number of diasterioisomers of which the a, b, and g forms predominate. During manufacture, g-HBCD is the most dominant diastereoisomer formed, contributing approximately 80% of the technical formulation.
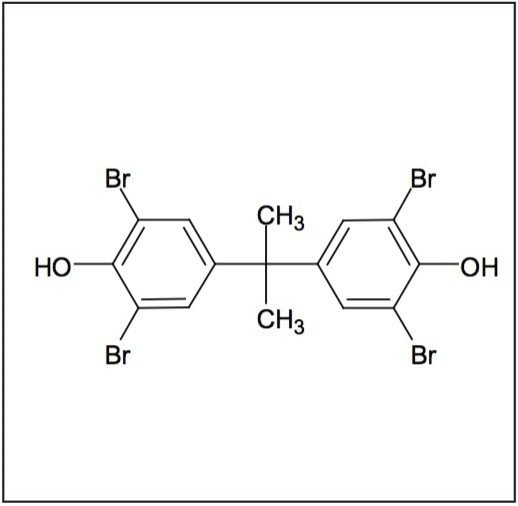
TBBP-A (Figure 2) is used to improve the fire safety of electrical and electronic quipment. It is the largest volume BFR for this application in production today.
Both HBCD and TBBP-A are currently marketed around the world without any legislative restrictions. However, as emerging contaminants or Persistent Organic Pollutants (POPs), the importance of continuous monitoring to quantify the impact of these chemicals on human health and the environment is paramount.
There is current concern as to the persistent nature of these chemicals and consequently, the detrimental effects they may have. Various studies have shown a presence of HBCD and TBBP-A in the environment as well as in aquatic and human tissues. There are indications that the residue levels of HBCD have increased significantly between 1969 and 1997.1,2
At their Twenty-third Meeting in December 2006, the Advisory Committee on Hazardous Substances (ACHS) highlighted the following problems with HBCD3:
There are robust methodologies reported in the literature4,5 for the analysis of TBBP-A and HBCD diastereomers using HPLC-MS/MS. However, advantages can be gained by the use of Waters UltraPerformance Liquid Chromatography (UPLC) through enhanced chromatographic resolution and throughput of the analytical method.6
In this application note, we describe a UPLC-MS/MS analysis of all five diastereomers of HBCD and TBBP-A in samples of marine origin. The optimized separation used in this method resulted in a run time of 10 minutes, with the five HBCD diastereomers and TBBP-A analyzed being separated to <10% valley.
Comparability of the UPLC data for real samples to that run using a standard HPLC-MS/MS validated method is excellent. The run time reduction of up to 15 minutes using UPLC offers a throughput improvement of up to five times, combined with a superior separation of target analytes.
Extracts of marine origin and calibration solutions containing BFRs and 13C-Labelled BFRs (internal standards) were provided by the Central Science Laboratory, (CSL) York, UK. The a, b, g, d, e HBCD and TBBP-A single compound standards were supplied by Wellington Laboratories. The methodology used for sample preparation and HPLC-MS/MS analysis are described elsewhere.8
|
LC system: |
ACQUITY UPLC |
|
Column: |
ACQUITY UPLC BEH C18 2.1 x 150 mm, 1.7 μm |
|
Column temp.: |
60 °C |
|
Flow rate: |
500 μL min-1 |
|
Mobile phase A: |
Water |
|
Mobile phase B: |
Methanol |
|
Total run time: |
10 min |
|
Injection volume: |
10 μL, Full loop injection |
|
Time(min) |
%A |
|---|---|
|
0.00 |
20 |
|
5.00 |
20 |
|
6.00 |
0 |
|
8.00 |
0 |
|
8.10 |
20 |
|
MS system: |
Quattro Premier XE |
|
Ionization mode: |
ESI |
|
Capillary voltage: |
2.5 kV |
|
Desolvation gas: |
Nitrogen, 1000 L/Hr, 400 ˚C |
|
Cone gas: |
Nitrogen, 20 L/Hr |
|
Source temp.: |
120 °C |
|
Acquisition: |
Multiple Reaction Monitoring (MRM) |
|
Transitions: |
See Table 1 |
|
Collision gas: |
Argon at 3.5 x 10-3 mBar |
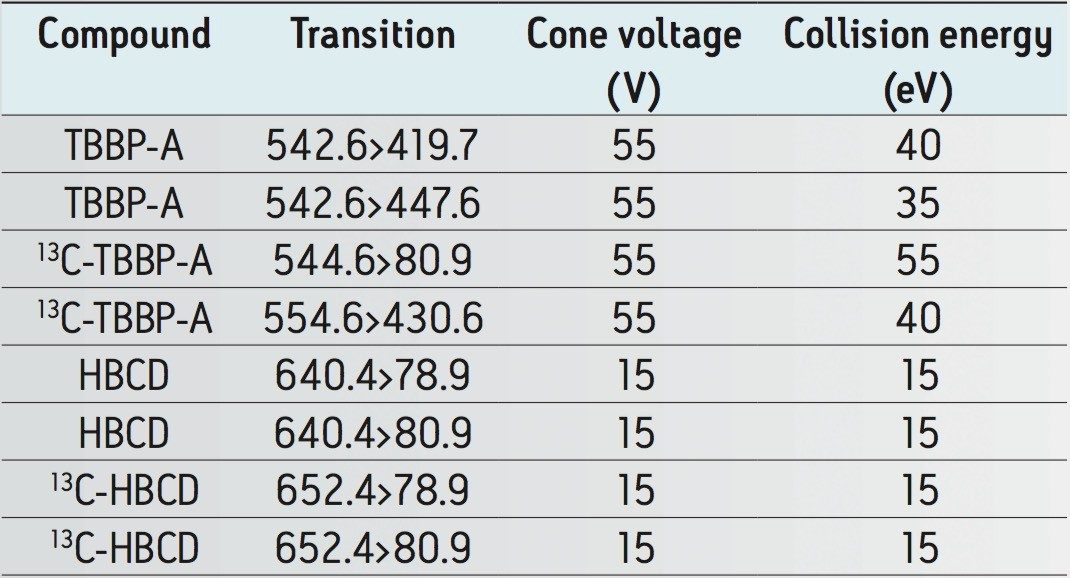
The data were acquired using Waters MassLynx Software v. 4.1. The data was processed using TargetLynx Application Manager. This quantification package is capable of automating quality control checks such as calculating ion ratios, flagging analytical results above/below thresholds set by the user, and many other features.
Current HPLC based methods are becoming more isomer specific7,8 to enable more specific toxicological studies to be performed.
Until recently, published HBCD concentration data have been derived by Gas Chromatographic (GC) techniques.
However, GC analysis is currently limited as it is unable to chromatographically resolve the different diastereoisomers using standard GC parameters. The diastereomers are thermally labile, with degradation or interconversion observed at temperatures >160 °C. Thus, values have been reported as total HBCD.
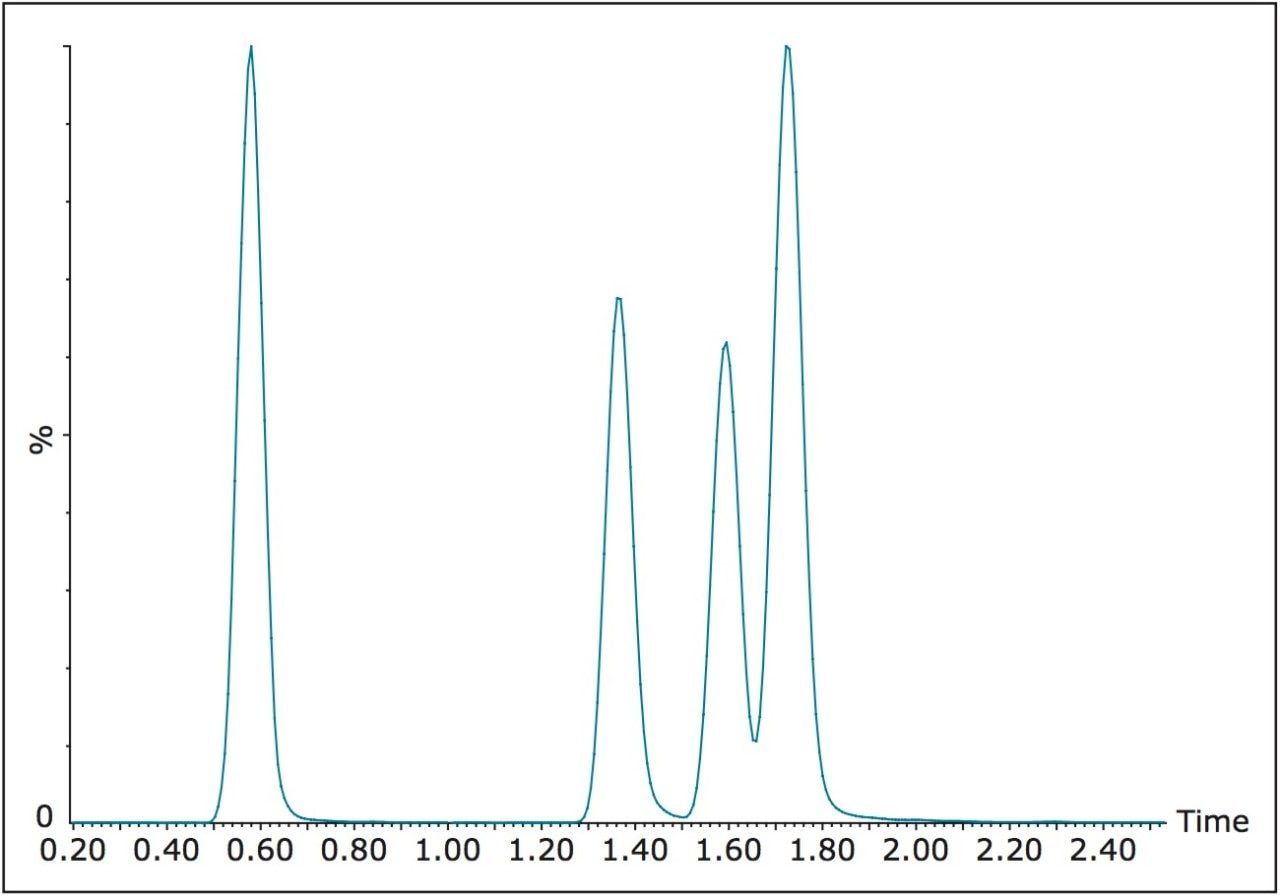
During development, two UPLC methods were assessed with comparisons being made to the original HPLC based method. The initial evaluation was a direct transfer of the separation achieved with HPLC. The chromatogram for the four target compounds (TBBP-A, a, b and g HBCD) using HPLC separation is shown in Figure 3.
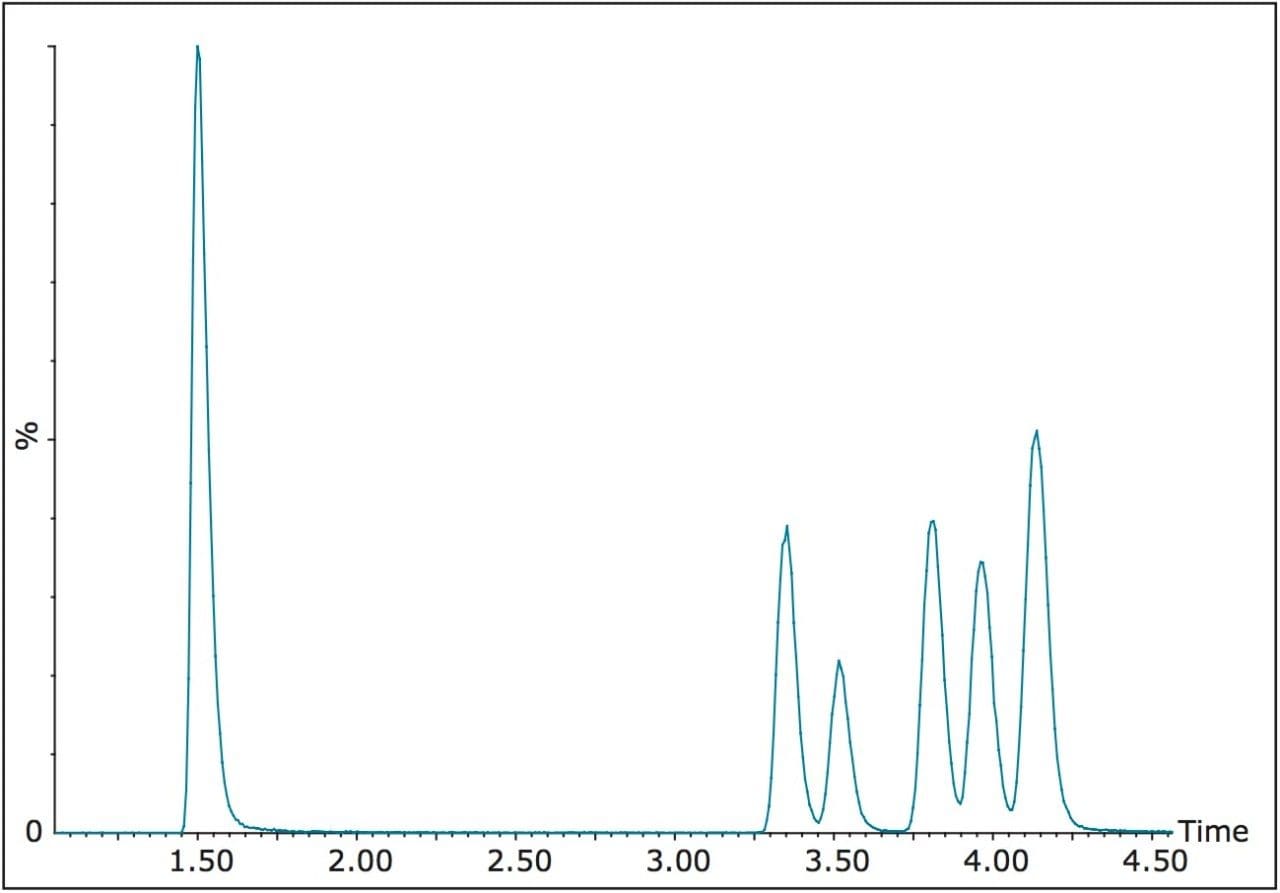
It was possible to achieve slightly improved chromatographic resolution, while reducing the total run time from 25 minutes to 5 minutes by using an ACQUITY UPLC BEH C18 Column, 130Å, 1.7 μm, 2.1 x 50 mm, part no. 186002350. The chromatograms for the optimized UPLC separation are shown in Figure 4. It can be seen that the UPLC separation would allow the laboratory to increase throughput from 2.4 samples per hour to 12 samples per hour.
After optimization of the rapid separation, a mixed standard containing the five HBCD diastereomers and TBBP-A was analyzed. To achieve the required separation, the UPLC BEH C18 150 x 2.1 mm, 1.7 μm column was required, which resulted in a total run time of 10 minutes (6 samples per hour).
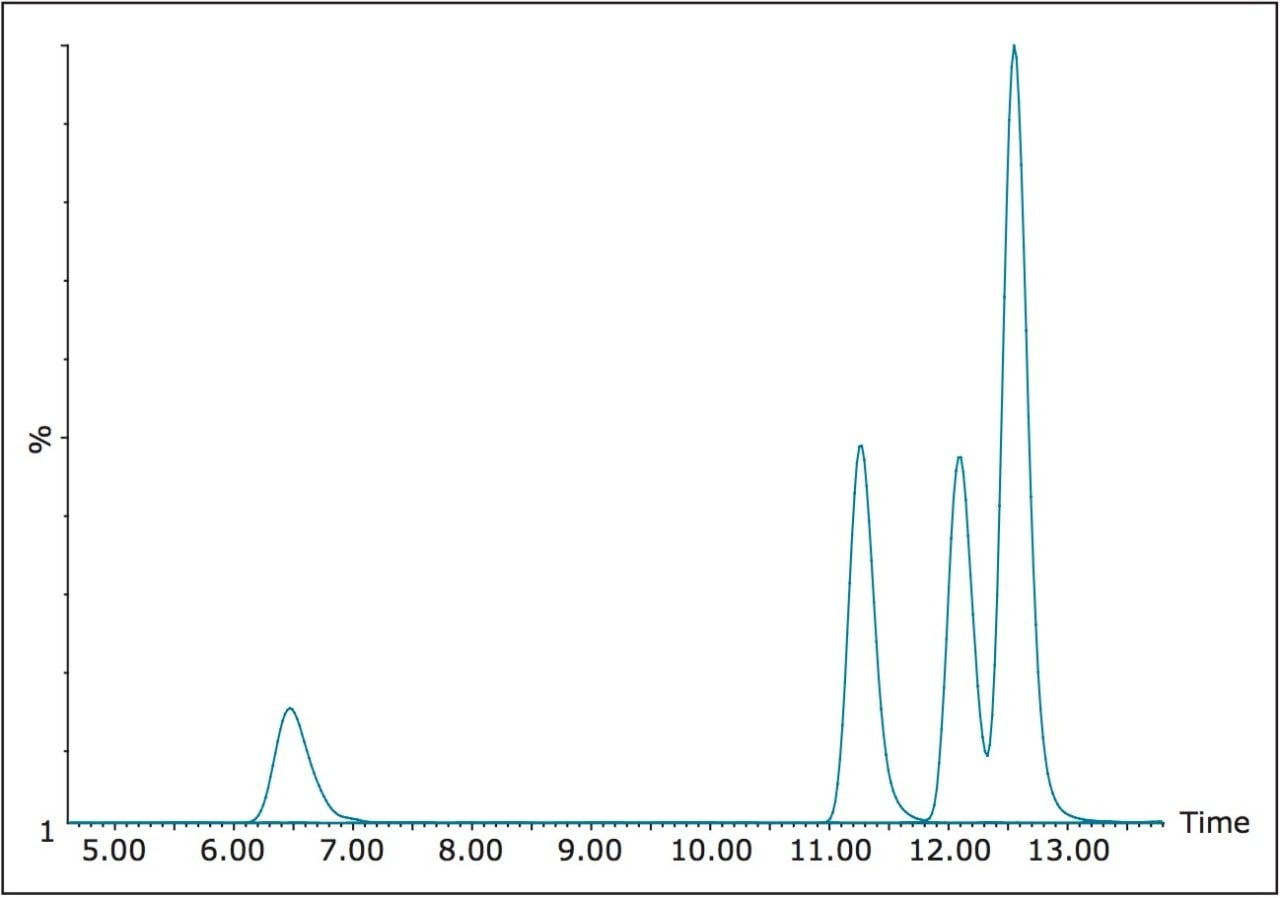
Acquisition of the five single component HBCD standards resulted in the elution order of a, b, g, d, and e being deduced, with peak widths of 0.15 minutes. The chromatogram for the eluting peaks, including TBBP-A is presented in Figure 5, where the valley of <10% between d and e HBCD can be observed.
Following this analysis, a bracketed calibration curve was acquired with a number of sample extracts. This curve contained both native and 13C-labelled a, b, g-HBCDs and TBBP-A, with native concentrations from 5 ng mL-1 to 600 ng mL-1.
The linearity of measurement over the calibration curve range was good for the four compounds determined quantitatively (TBBP-A, a, b, g-HBCDs), with all coefficients of determination (r2) being >0.999 for the un-weighted curves.
The reduction in peak widths achieved using UPLC separation resulted in significant sensitivity gains when compared with HPLC separation, with the TBBP-A peak width being reduced from 0.67 minutes to 0.15 minutes and the HBCDs peak widths being reduced from 0.53 minute to 0.16 minute. The chromatograms in Figure 6 show the comparative signals for the target compounds for a 10 ng mL-1 solvent standard.
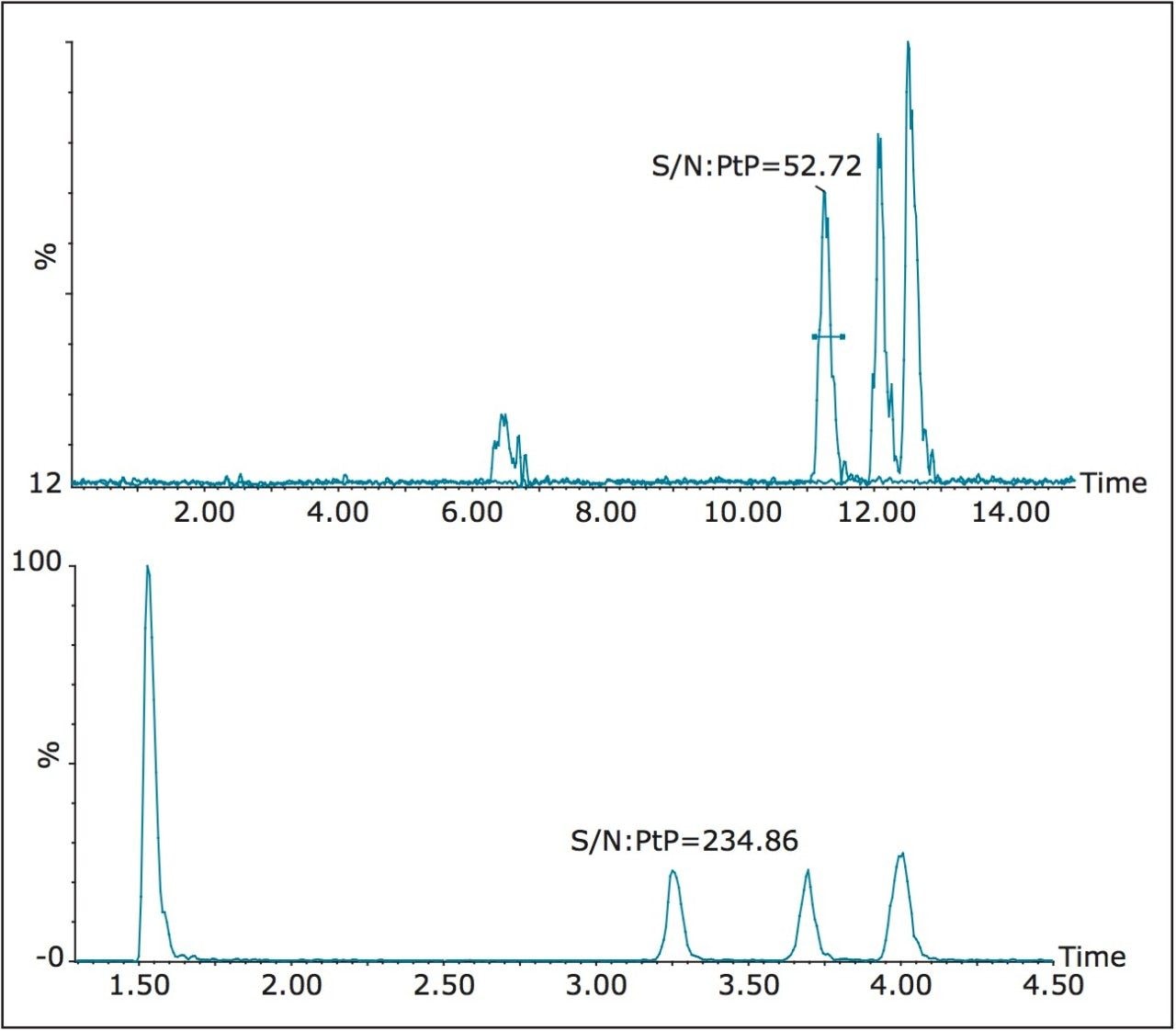
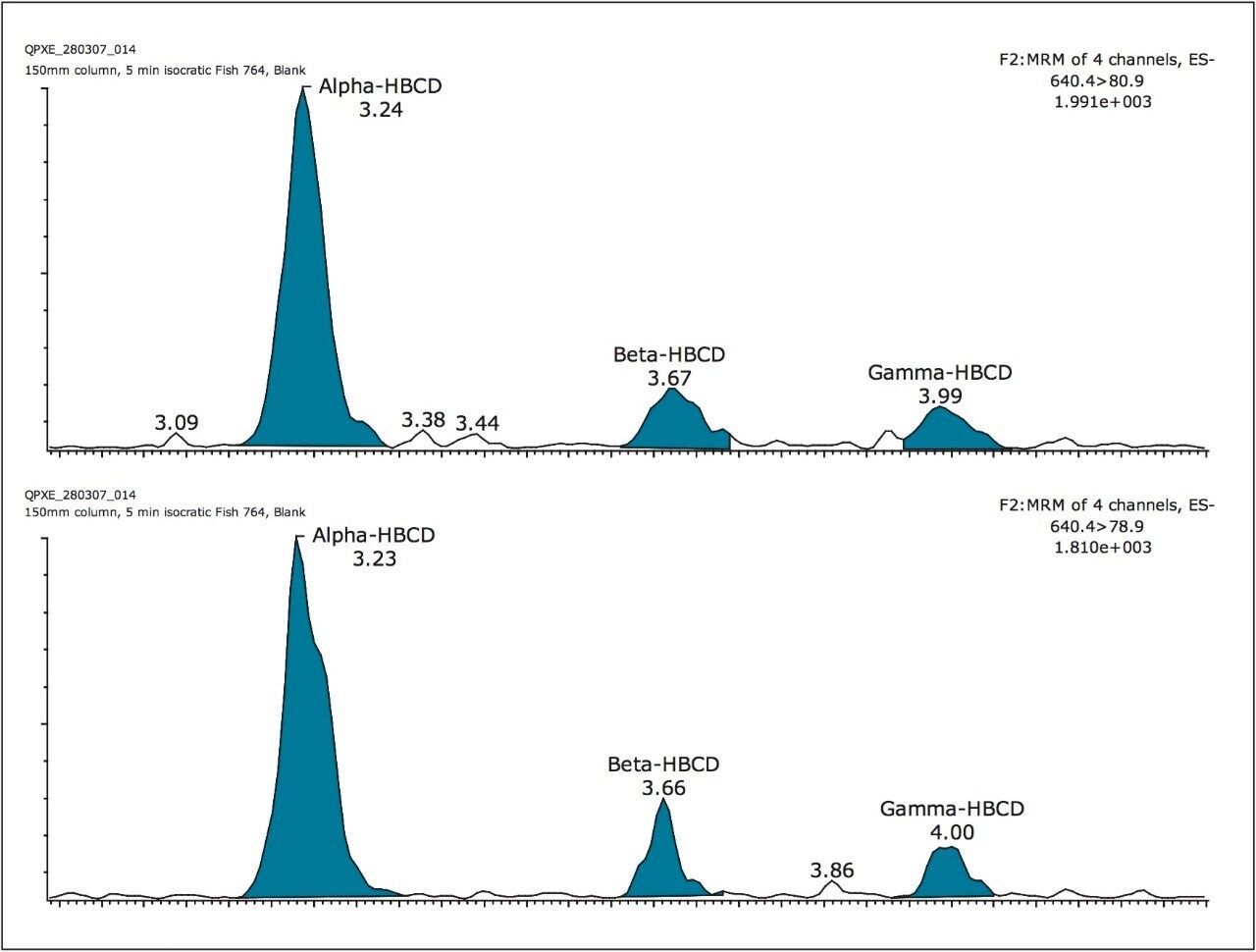
TBBP-A was detected in all of the marine origin samples analyzed, with a, b, g-HBCDs being detected in most extracts, d and e-HBCD were not detected in any. The a-enantiomer dominated in all the samples analyzed, as observed in other reports.5,7 This profile is characteristic of marine biota and probably arises as a result of selective metabolism of the different enantiomers and/or biotransformation processes.
A typical marine origin extract with concentrations of 0.38, 0.056, and 0.032 ng g-1 for a, b, g-HBCD respectively is given in Figure 7.
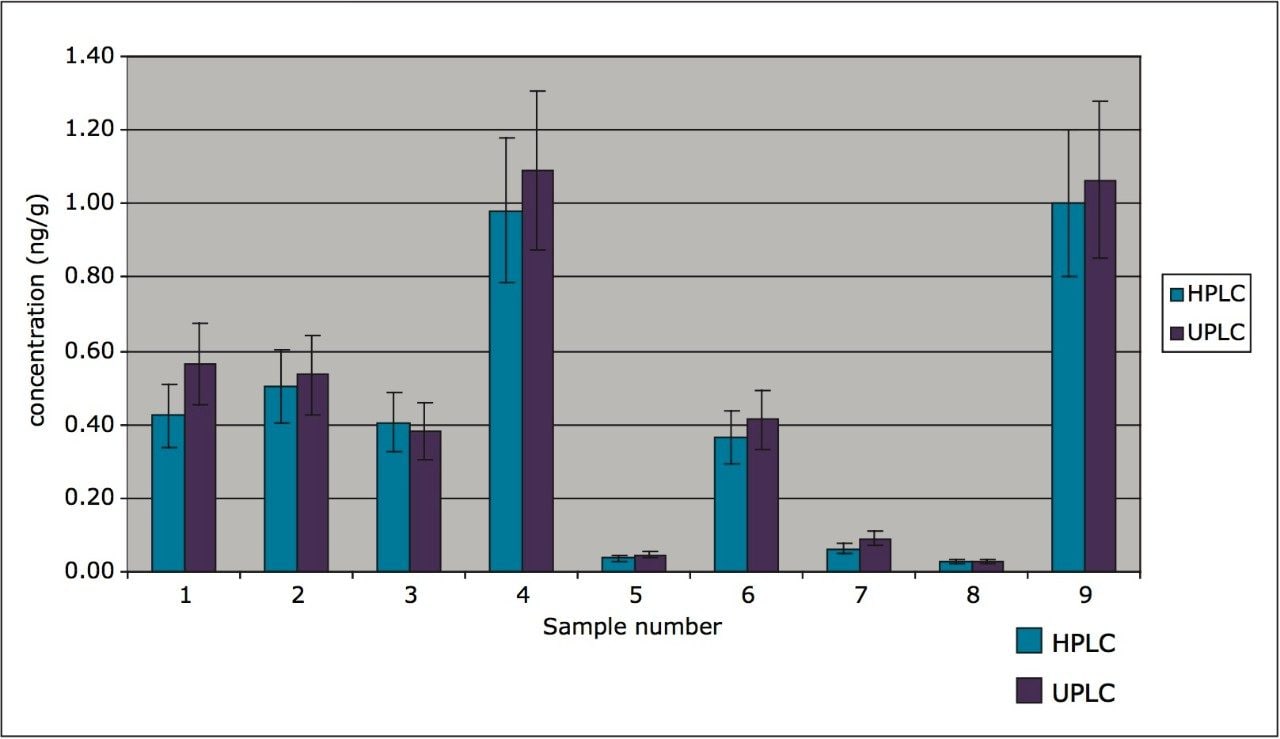
All results were compared between the two methods. The mean deviation between HPLC and UPLC was <20%. a-HBCD results across the batch are shown in Table 2, with all data shown with 15% error bars, conforming with a typical measurement uncertainty.
The use of the ACQUITY UPLC System with tandem (triple) quadrupole mass spectrometry enabled a significant improvement in chromatographic resolution and a reduction of run time over current HPLC methods.
All five HBCD diastereomers and TBBP-A could be determined rapidly, with added confidence given through TargetLynx data processing.
This vastly increases a cost conscious laboratory’s productivity by reducing both run time and acquisition-to-report time. Also, cost and environmental impact will be reduced through lower solvent usage required with UPLC.
Final results compare favorably with an established fully validated (ISO 17025) method ensuring confidence in results.
This methodology carried out on the Waters ACQUITY UPLC System with tandem (triple) quadrupole mass spectrometry can enable laboratories to achieve increased sample capacity, flexibility in workflow, and improved lab efficiency, leading to maximized asset utilization and a faster return on investment.
720002445, February 2016