This is an Application Brief and does not contain a detailed Experimental section.
The aim of this technology brief is to gather more data during routine analytical testing.
Using modernized methods in regulated environments can save both time and money. Combining these modernized methods with advanced instrumentation such as the ACQUITY QDa Detector can provide additional benefits, including detecting important compounds that do not have UV absorbing chromophores. Taking advantage of the best equipment and modern analytical methods can provide additional information quicker, leading to faster and more informed decision making.
Using CORTECS C8 Columns with the ACQUITY QDa Detector for analysis of additives in pharmaceuticals provides additional information quicker, leading to faster and more informed decision making.
Manufacturing pharmaceutical products consists of many stages. Besides adding the required amount of active pharmaceutical ingredients (API), the formulated drug usually requires non-active pharmaceutical ingredients (NAPIs) as excipients to control product physical properties. In formulated liquid products, such as cold and cough syrups, common additives are polyethylene glycols (PEGs), which help give these syrups their unique texture. The FDA requires monitoring of APIs and NAPIs during pharmaceutical production but ingredients like PEGs are difficult to analyze since they lack a UV absorbing chromophore.
|
LC system: |
ACQUITY UPLC H-Class System |
|
Mass detection: |
ACQUITY QDa Detector |
|
UV detection: |
ACQUITY UPLC PDA Detector (280 nm) |
|
Columns: |
CORTECS C8 Column, 2.7 μm, 3 x 100 mm |
|
Column temp.: |
30 °C |
|
Sample temp.: |
Ambient |
|
Injection volume: |
1 μL |
|
Flow rate: |
0.6 mL/min |
Using a mass spectrometer is one way to detect NAPIs that do not have a chromophore. However, mass spectrometers often require a significant amount of training and equipment to operate properly. The ACQUITY QDa Detector is a user friendly mass spectrometer requiring very little training to operate. Using an ACQUITY QDa Detector can provide additional information during analytical runs that may not be accessible without the use of more expensive and complex instruments.
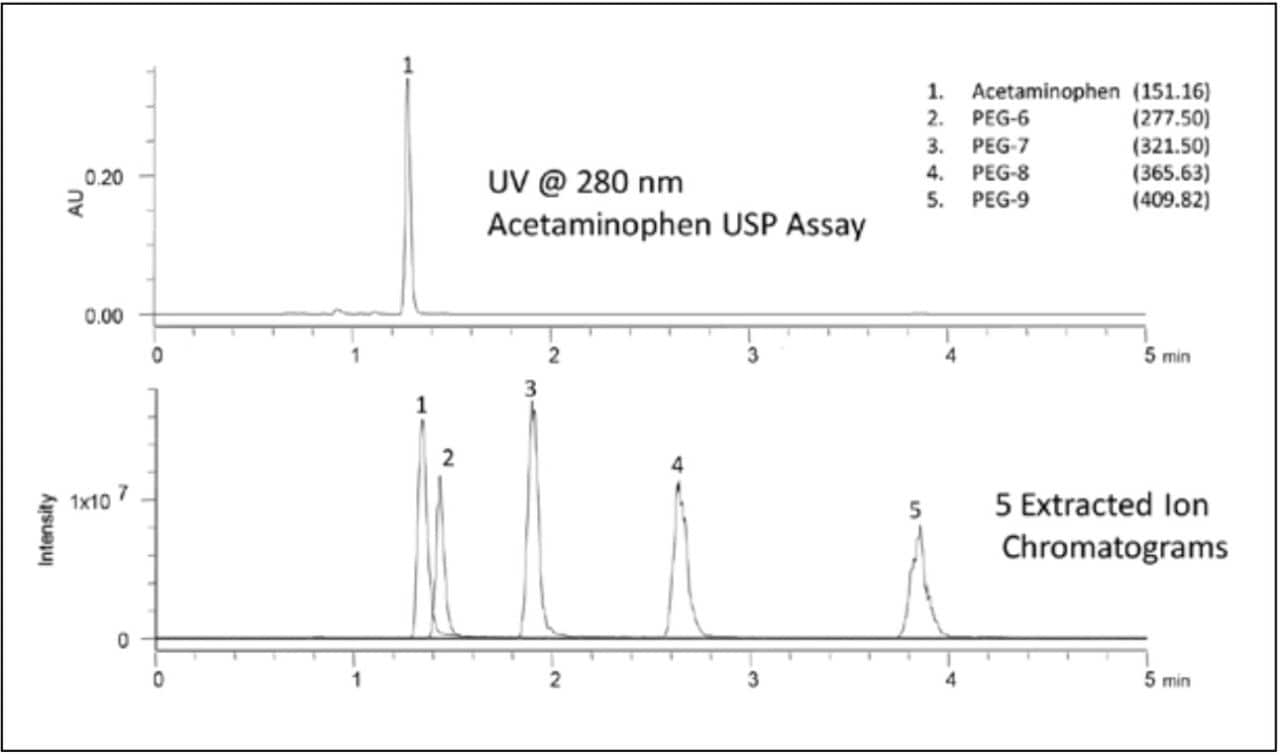
Figure 1 shows an example of an analysis that gained additional information from the use of an ACQUITY QDa Detector. The USP assay test for Acetaminophen in cough syrup was transferred to facilitate a faster analysis of the sample. Following USP General Chapter <621> guidelines, the original method using a 4.6 x 150 mm L7 column was transferred to a CORTECS C8 Column, 2.7 μm, 3 x 100 mm (p/n 186008361). By modernizing the compendial method, a three-fold decrease in run time is achieved. The separation is performed isocratically using water:methanol (80:20) with 1% glacial acetic acid and UV monitoring at 280 nm. The ACQUITY QDa Detector was set to scan, in the positive mode, a range of masses from 50–1,000 Da, using a cone voltage of 15 V, and a capillary voltage of 1.5 kV.
As Figure 1 shows, the UV detector only allows monitoring of the API, whereas the ACQUITY QDa Detector visualizes and confirms identity for the API plus four PEGs. Using the most advanced column technology, CORTECS Columns, and advanced instrumentation such as the ACQUITY QDa Detector, can lead to a better understanding and faster results.
Modernization of older USP monograph tests can lead to a variety of benefits, including reduced sample turnaround time, increased separation performance, and reduced solvent and sample consumption. USP General Chapter <621> outlines acceptable changes to isocratic method conditions and should be consulted when updating older monographs. Using modernized methods in regulated environments can save both time and money. Combining these modernized methods with advanced instrumentation such as the ACQUITY QDa Detector can provide additional benefits, including detecting important compounds that do not have UV absorbing chromophores. Taking advantage of the best equipment and modern analytical methods can provide additional information quicker, leading to faster and more informed decision making.
720005576, January 2016