For forensic toxicology use only.
This application note describes a rapid and broadly applicable SPE protocol and UPLC-MS/MS method for analyzing a comprehensive panel of compounds common to forensic toxicology screens. The unique, water-wettable nature of the Oasis sorbent allowed us to eliminate the common conditioning and equilibration steps without any loss in recovery or reproducibility for any of the 38 compounds in this testing panel. This property also enables the entire hydrolysis step to be conducted within the wells of the Oasis MCX μElution Plate, eliminating the time-consuming and error-prone transfer steps. Combining this with the consolidation of two wash steps into a single one further facilitates the reduction of a six-step extraction process into only three steps. Though this procedure remains slightly more timeconsuming than sample dilution, it nonetheless can be completed in 30 minutes. Moreover, it offers the additional added benefits of increased sensitivity, reduced matrix interferences, increased analytical column lifetimes, and reduced risk of ion source-fouling.
This method was designed for the analysis of enzymatically hydrolyzed samples. Yet the use of the ACQUITY UPLC BEH Phenyl Column also enables the resolution and analysis of morphine-3-glucuronide and morphine-6-glucuronide, allowing the method’s use for direct analysis without hydrolysis. It also allows monitoring of the metabolites of these glucuronides. Because these compounds have been shown to be difficult to fully hydrolyze using beta glucuronidase,2 monitoring their presence can be an important factor in ensuring complete conversions to the free drugs.
This method enables the rapid extraction and analysis of a large panel of drugs for forensic toxicology screening. When combined with the chromatography of the ACQUITY UPLC BEH Phenyl Column, it provides a rapid, specific method with the sensitivity and reproducibility required to accurately screen for this panel of compounds.
In forensic toxicology, drug-screening panels often include commonly used substances such as opiates, benzodiazepines, and stimulants. Often, multiple screening methods are used to obtain a comprehensive view of the multiple drug classes. These methods may include immunoassay, GC-MS, LC-MS/MS, or a combination of methods. Regardless of the methods used, the goal is to achieve sufficient sensitivity, specificity, and accuracy to proceed with the appropriate confirmation, or alternatively, to be confident a sample tests negative.
Sample preparation is as important a consideration in forensic toxicology screening as the choice of instrumentation technique. While many laboratories use a “dilute and shoot” approach for urinary toxicology panels, the presence of matrix components, buffers, residual enzymes, and other substances in the sample can result in excessive matrix effects, significantly reduced column lifetimes, and increased instrument downtime resulting from contaminant buildup on electrospray sources in LC-MS.
Though solid phase extraction (SPE) is often perceived as difficult or time-consuming, a judicious choice of method can simplify this process significantly. The most selective method of sample preparation, SPE results in cleaner samples than most other techniques, making it ideal for obtaining accurate results.
Here we detail a single sample preparation and UPLC-MS/MS analysis strategy for a comprehensive panel of compounds often analyzed in forensic toxicology screens. In an abbreviated, modified extraction method, Waters’ Oasis MCX μElution Plates are used to rapidly extract a panel that includes opioids, amine stimulants, benzodiazepines, benzoylecgonine (BZE), and phencyclidine (PCP). UPLC-MS/MS analysis is achieved using a Waters ACQUITY UPLC BEH Phenyl Column and a Xevo TQD. All sample preparation steps, including enzymatic hydrolysis, are performed within the wells of the micro-elution plates, and the extraction method is simplified, eliminating conditioning and equilibration steps, and consolidating the wash procedures into a single step.
All standards were obtained from Cerilliant (Round Rock, TX). Stock solutions were prepared in methanol. Samples were prepared by diluting stock solutions into pooled, blank urine. All analytes are listed in Table 1.
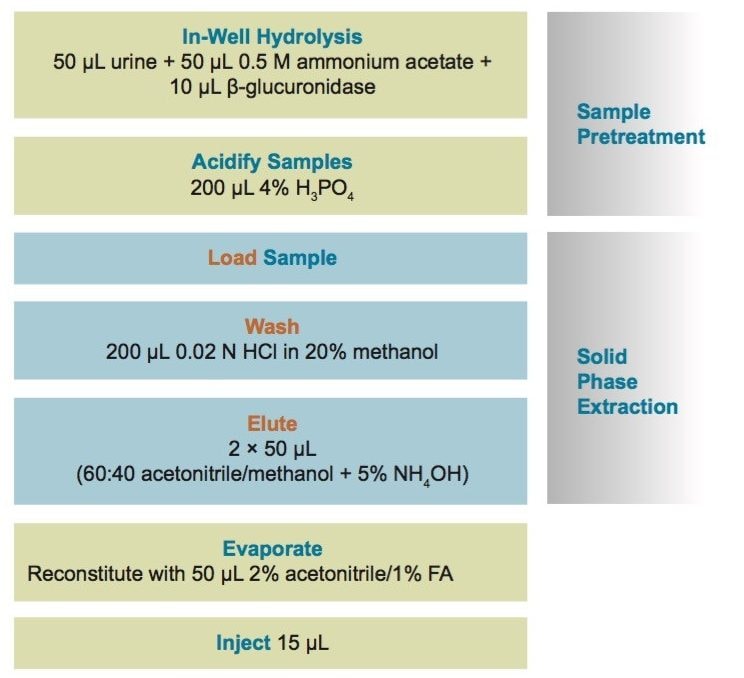
50 μL of urine was added to individual wells of an Oasis MCX μElution Plate (p/n 186001830BA), along with 50 μL of 0.5 M ammonium acetate buffer and 10 μL of β-glucuronidase enzyme (Roche, E. coli) to simulate all the reagents added for enzymatic hydrolysis. 200 μL of 4% H3PO4 was then added and each sample was mixed by several aspirations. The samples were then drawn into the sorbent bed by vacuum. All samples were subsequently washed with 200 μL of 20% MeOH containing 0.02 N HCl. After washing, the plate was dried under high vacuum (~15 inches Hg) for 5–10 minutes, to remove as much of the wash solution as possible. Samples were then eluted with 2 x 50 μL of 60:40 ACN:MeOH containing 5% strong ammonia solution (Fisher, 28–30%). All samples were evaporated to dryness under nitrogen at 40 °C and reconstituted with 50 μL of sample diluent (2% ACN:1% formic acid in MilliQ water). Figure 1 depicts the workflow of the extraction procedure.
|
LC system: |
ACQUITY UPLC I-Class (FL) |
|
Column: |
ACQUITY UPLC BEH Phenyl, 1.7 μm, 2.1 x 100 mm (p/n 186002352) |
|
Column temp.: |
40 ˚C |
|
Sample temp.: |
10 ˚C |
|
Injection vol.: |
15 μL |
|
Flow rate: |
0.6 mL/min |
|
Mobile phase A (MPA): |
0.1% formic acid in MilliQ water |
|
Mobile phase B (MPB): |
0.1% formic acid in acetonitrile (ACN) |
|
Gradient: |
Initial conditions were 95:5 MPA/MPB. The percentage of MPB was increased to 62.5% over 5 minutes, returned to 5% over 0.1 minutes, and remained at 5% for 0.9 minutes. The entire cycle time was 6.0 minutes. |
|
MS system: |
Xevo TQD |
|
Ionization mode: |
ESI positive |
|
Acquisition range: |
MRM transitions optimized for individual compounds |
|
Capillary voltage: |
1.0 kV |
|
Collision energy: |
Optimized for individual compounds (see Table 2) |
|
Cone voltage: |
Optimized for individual compounds (see Table 2) |
MassLynx with TargetLynx Application Manager
Analyte recovery was calculated according to the following equation:
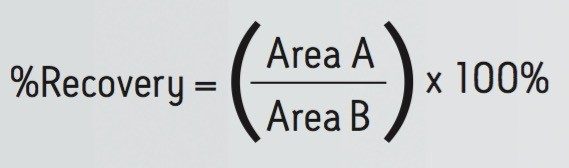
Where A = the peak area of an extracted sample and B = the peak area of an extracted matrix sample to which the compounds were added post-extraction.
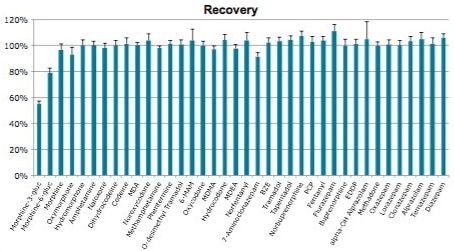
All test compounds are listed in Table 1, and Figure 2 shows their chromatography. The compounds are grouped into related classes to facilitate viewing. Table 2 lists the retention times and MS conditions of all compounds in their elution order. Previous work1 demonstrates the increased ability, compared with other columns, of the ACQUITY UPLC BEH Phenyl Column to retain polar opiate compounds. Combined with the narrow peak shape of UPLC, this chromatographic method retains and separates even the most polar analytes, maintaining the resolution of isobaric compounds, while still allowing all compounds to elute within four minutes. Baseline resolution was readily obtained between all isobaric groups, including morphine-3-glucuronide, hydromorphone-glucuronide, and morphine-6-glucuronide, supporting the ability to accurately identify and quantify all compounds. Such resolution is useful for monitoring these compounds to ensure complete hydrolysis or in cases where direct quantification of these metabolites is desired. Baseline separation was also achieved between methamphetamine and phentermine, which share a major product ion and can interfere with each other.
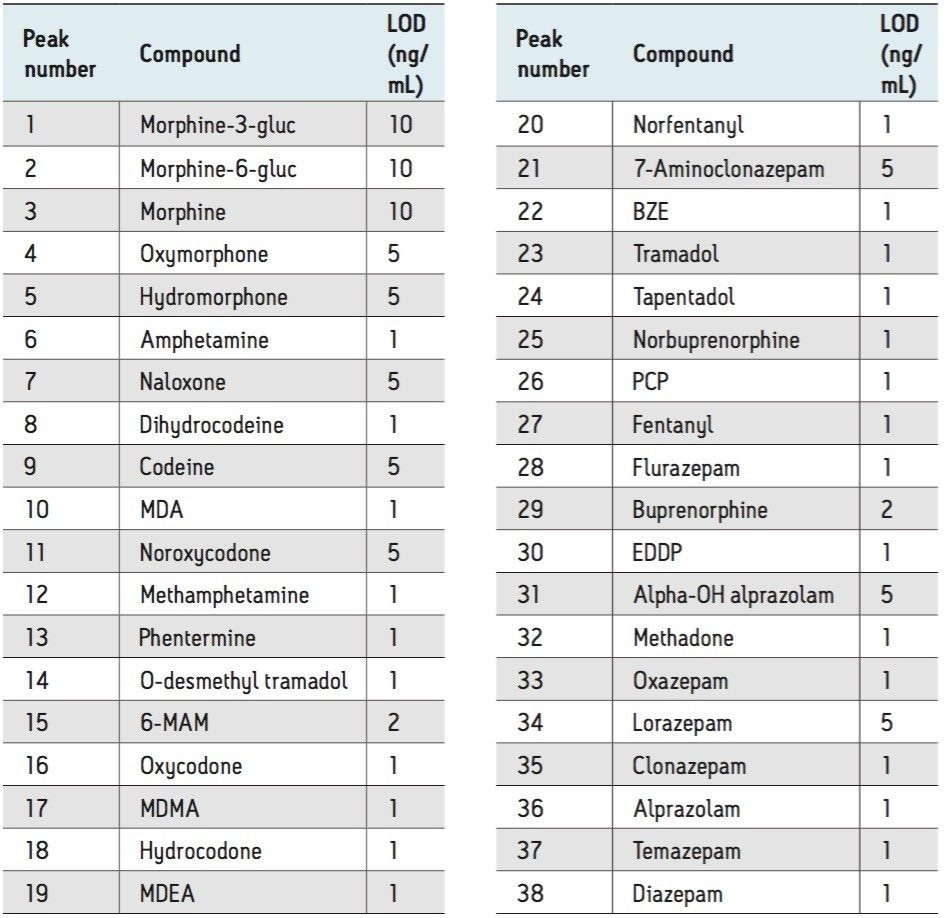
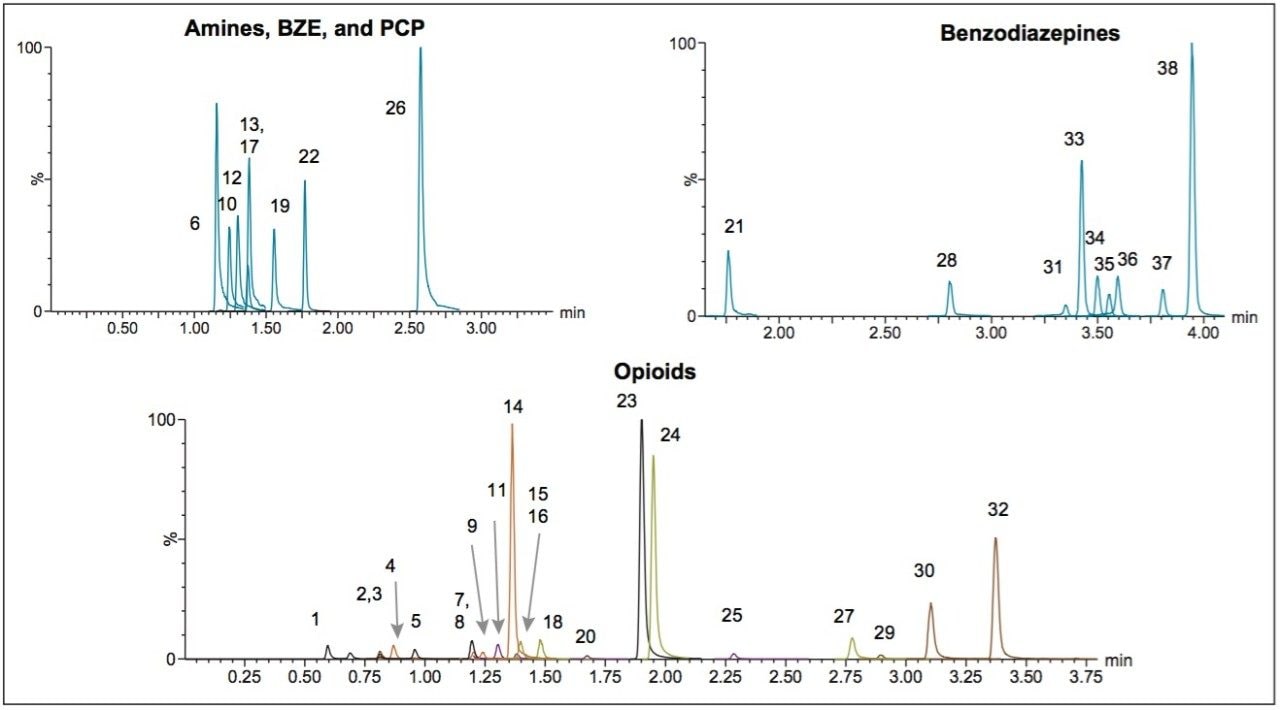
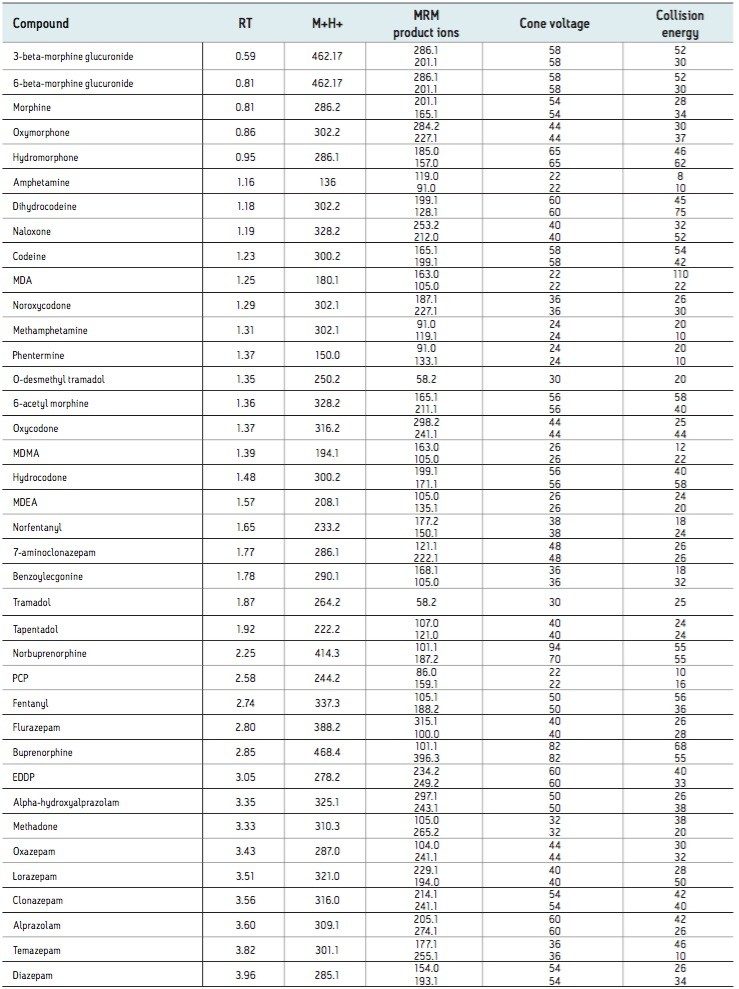
Figure 3 shows the extraction recoveries of the entire panel of compounds. With the exception of morphine-3-glucuronide, all compounds had recoveries greater than 80%. The average recovery was 100% for all compounds. Extraction efficiencies were also consistent. Coefficients of variation (%CV) were less than 10% for 37 of 38 compounds and only 12.5% for the remaining compound. A series of experiments performed during method development revealed that more than 20% methanol in the wash step resulted in loss of the acidic benzodiazapines, such as oxazepam, clonazepam, lorazepam, and temazepam. Thus, the single wash step consisted of 20% methanol containing 0.02 N HCl. This simple modification enabled the highly efficient extraction of the entire panel of compounds in a single method.
In addition to the benefit of extracting multiple drug classes using a single SPE method, the traditional six-step mixed-mode SPE method was simplified into just three steps. The conditioning and equilibration steps were eliminated, and the two wash steps (aqueous and organic) were combined into one. Eliminating these steps did not effect the extraction efficiency of the method (data not shown), a result consistent with the water-wettable nature of the sorbent. Unlike traditional silica-based sorbents, Oasis sorbent does not lose retentivity if allowed to dry out. This property also enables all sample pretreatments to be performed within the wells of the 96-well plate, eliminating individual transfer steps that can be time-consuming or error-prone. Combining the wash steps into a single wash also helps to accelerate the workflow. Compared to a traditional mixed-mode SPE workflow, which includes conditioning, equilibration, and two wash steps, half of these steps are eliminated. This reduces a six-step procedure to a three-step procedure, reducing processing time by 50%. An entire plate can be processed within 30 minutes.
Five-point calibration curves at 1, 5, 10, 50, and 100 ng/mL (0.2, 1.0, 2.0, 10, and 20 ng/mL for fentanyl, norfentanyl, 6-acetyl morphine, norbuprenorphine, and buprenorphine) were extracted in order to estimate limits of detection for the assay. Limits of detection were defined as those points in which the signal was five times greater than that of an extracted matrix blank, and both bias and %CV were less than 20%. Table 1 shows the calculated LODs for each compound. These values ranged from 1 to 10 ng/mL depending on the compound. This demonstrates that the method has the sensitivity and specificity required for semi-quantitative screening of this expanded toxicology panel.
We have described a rapid and broadly applicable SPE protocol and UPLC-MS/MS method for analyzing a comprehensive panel of compounds common to forensic toxicology screens. The unique, water-wettable nature of the Oasis sorbent allowed us to eliminate the common conditioning and equilibration steps without any loss in recovery or reproducibility for any of the 38 compounds in this testing panel. This property also enables the entire hydrolysis step to be conducted within the wells of the Oasis MCX μElution Plate, eliminating the time-consuming and error-prone transfer steps. Combining this with the consolidation of two wash steps into a single one further facilitates the reduction of a six-step extraction process into only three steps. Though this procedure remains slightly more time-consuming than sample dilution, it nonetheless can be completed in 30 minutes. Moreover, it offers the additional added benefits of increased sensitivity, reduced matrix interferences, increased analytical column lifetimes, and reduced risk of ion source-fouling.
This method was designed for the analysis of enzymatically hydrolyzed samples. Yet the use of the ACQUITY UPLC BEH Phenyl Column also enables the resolution and analysis of morphine-3-glucuronide and morphine-6-glucuronide, allowing the method’s use for direct analysis without hydrolysis. It also allows monitoring of the metabolites of these glucuronides. Because these compounds have been shown to be difficult to fully hydrolyze using beta glucuronidase,2 monitoring their presence can be an important factor in ensuring complete conversions to the free drugs.
This method enables the rapid extraction and analysis of a large panel of drugs for forensic toxicology screening. When combined with the chromatography of the ACQUITY UPLC BEH Phenyl Column, it provides a rapid, specific method with the sensitivity and reproducibility required to accurately screen for this panel of compounds.
720005290, January 2015