This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates to separate enantiomers of 9-hydroxyrisperidone and apply this method to monitor the formation over a metabolic time course.
The combination of UPC2 with MS detection facilitates the enantiomeric separation of 9-hydroxyrisperidone.
Many drug candidates, as well as their metabolites, contain one or more chiral centers. During the course of the drug development process, an essential step is to identify and monitor the various enantiomers that may exist. Supercritical fluid chromatography (SFC) is known to be a highly effective technique for performing chiral separations. In addition, the use of SFC provides benefits such as high efficiency, fast separations, and use of solvents that are MS compatible.
In this technology brief, we demonstrate the enantiomeric separation and detection of 9-hydroxyrisperidone, the active metabolite of risperidone, utilizing UPC2-MS/MS. Method optimization was performed using 9-hydroxyrisperidone standards in solution, then was applied to samples where risperidone was incubated with human liver microsomes. The conversion of parent to the hydroxylated metabolite was monitored. Figure 1 shows the optimized chromatography for achiral risperidone and the R and S forms of 9-hydroxyrisperidone obtained using a Waters Trefoil CEL2 3 x 150 mm, 2.5-μm column, using ammonium formate modified methanol as the co-solvent.
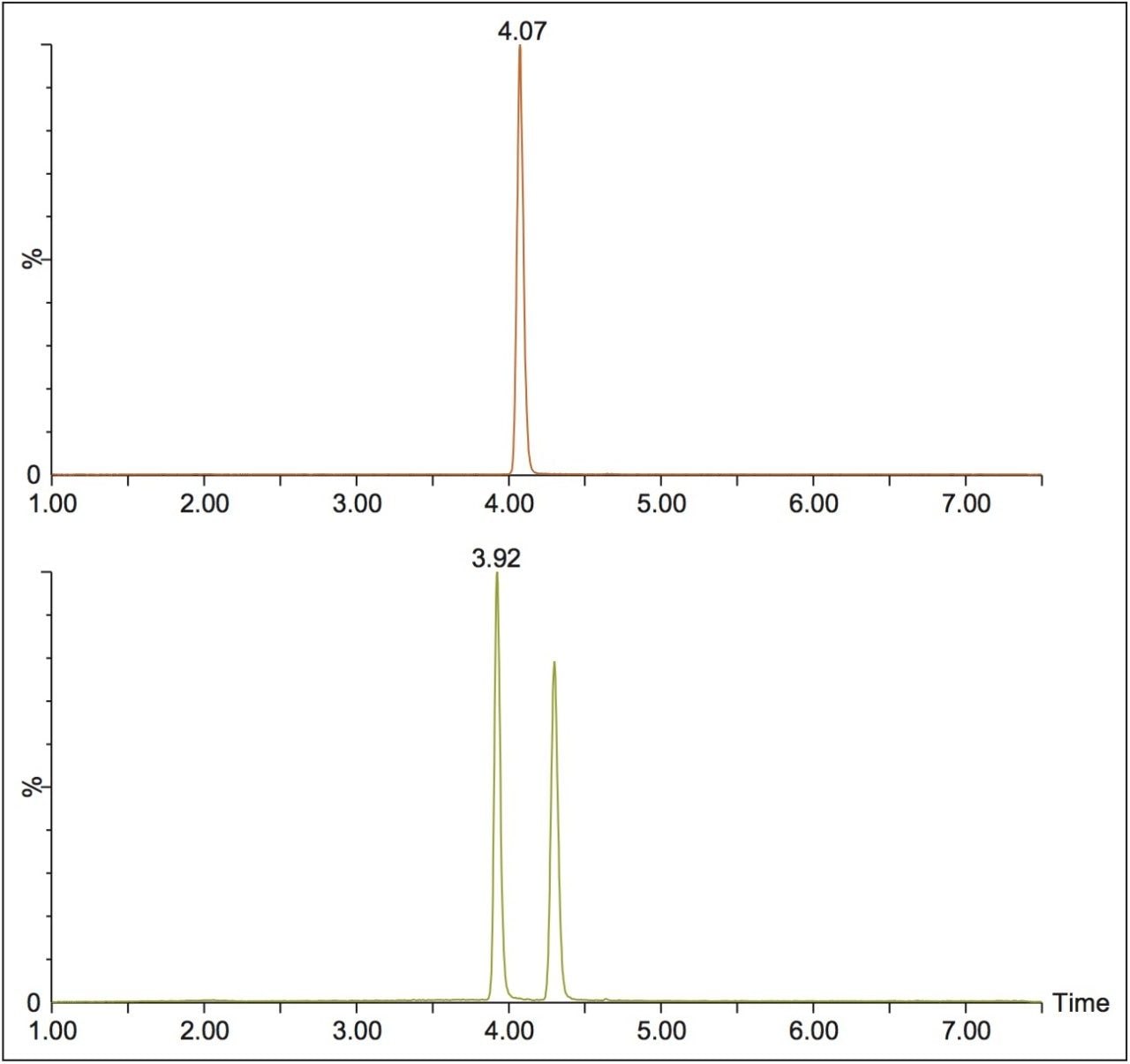
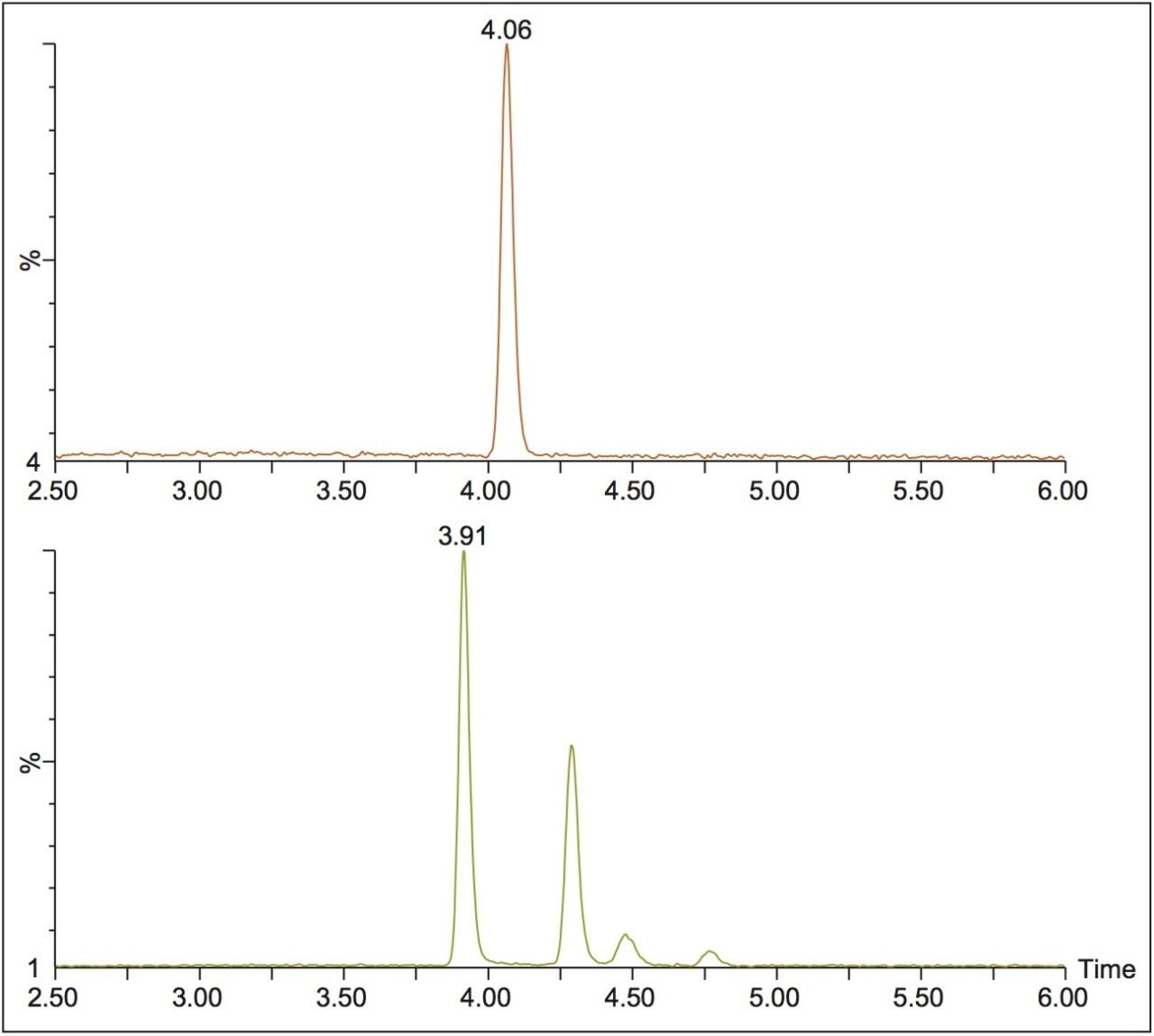
This method was then used to analyze incubated samples quenched at t = 0, 15, 30, 60, 90, and 120 min, to monitor the conversion of the parent risperidone to the 9-hydroxy metabolite. An example injection is shown in Figure 2, where the two expected peaks for the 9-hydroxy metabolite are seen at 3.92 and 4.29 min. However, two additional peaks are seen at 4.48 and 4.76 min, which could be attributed to the minor 7-hydroxy risperidone metabolite described in the literature.1
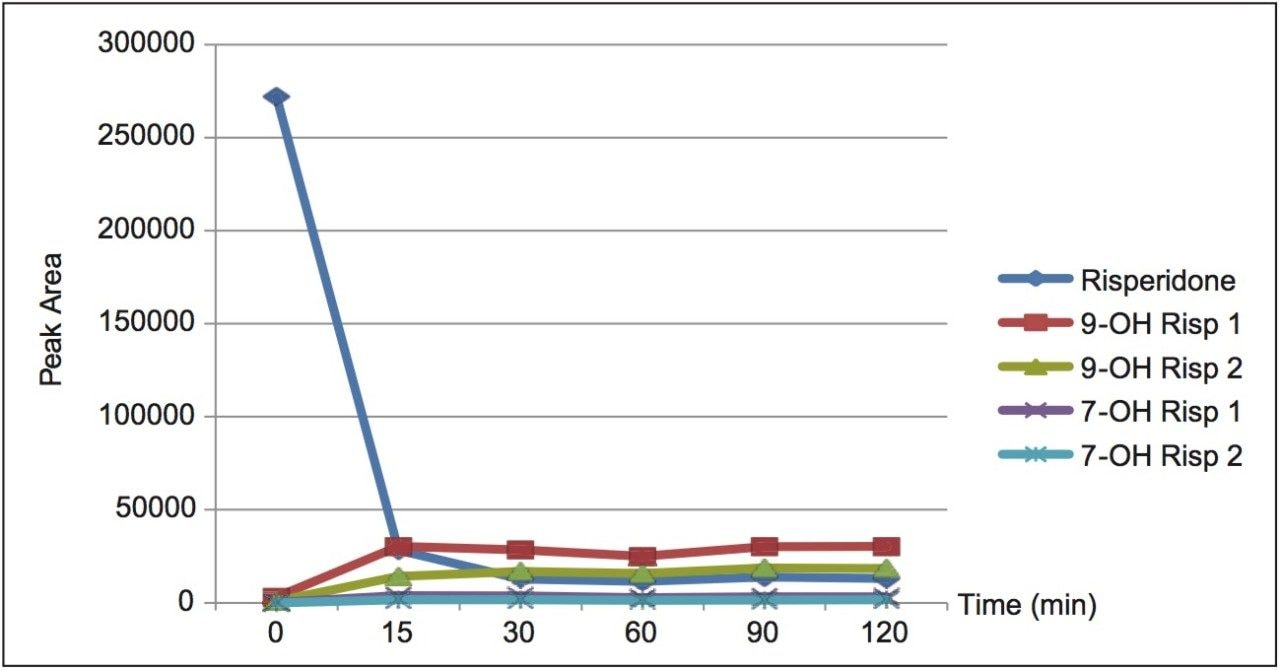
The peak areas obtained for the parent compound, risperidone, and the four hydroxylated metabolite peaks were plotted as a function of time. Figure 3 shows that the majority of parent conversion to metabolite has occurred by 30 min. While the absolute identity of the R and S forms were not confirmed, published data2 states that the formation of R-9-hydroxy risperidone is favored. We, therefore, postulate that the earlier eluting peak is the R form and the second peak is the S form.
The combination of UPC2 with MS detection facilitated the enantiomeric separation of 9-hydroxyrisperidone, which is the active metabolite of risperidone. The ability to isolate the various chiral forms of a compound is an essential step during the drug development process. Additionally, we have shown that convergence chromatography can be successfully used in metabolic stability studies.
720005181, September 2014