For forensic toxicology use only.
This application highlights a method for the analysis of 26 opioid drugs and metabolites by mixed-mode SPE followed by UPLC-MS/MS. Glucuronide metabolites are directly analyzed, eliminating the need for enzymatic or chemical hydrolysis.
The analysis of natural and synthetic opioid drugs continues to be an important aspect of forensic toxicology. In the past, analyses were typically conducted by GC-MS after first subjecting the samples to acid or enzymatic hydrolysis to liberate glucuronide metabolites.1 With the advent of LC-MS/MS techniques, glucuronide metabolites can now be analyzed directly.2-5 Direct analyses of these metabolites can eliminate the risk of false negatives due to incomplete hydrolysis, as enzymatic efficiency can vary greatly depending upon the enzyme used and the drug substrate analyzed.6
One particular sample matrix that has become increasingly popular recently is oral fluid. Unlike urine, oral fluid can be more indicative of current impairment or intoxication. Collection can also be easily accomplished without the privacy issues and adulteration possibilities associated with urine collection. Oral fluid also has similar advantages over blood as a matrix. Once again, collection is much easier, since it is non-invasive and there is no need for specialized training. This application highlights a method for the analysis of 26 opioid drugs and metabolites by mixed-mode SPE followed by UPLC-MS/MS. Glucuronide metabolites are directly analyzed, eliminating the need for enzymatic or chemical hydrolysis.
|
LC conditions |
|
|---|---|
|
LC system: |
ACQUITY UPLC I-Class |
|
Column: |
ACQUITY UPLC BEH C18 1.7 μm; 2.1 x 100 mm (p/n 186002352) |
|
Column temp.: |
30 °C |
|
Injection volume: |
10 μL |
|
Flow rate: |
0.4 mL/min. |
|
Mobile phase A: |
0.1% formic acid in MilliQ water |
|
Mobile phase B: |
0.1% formic acid in ACN |
|
Weak needle wash: |
2% ACN in water |
|
Strong needle wash: |
10% ACN in water |
|
Gradient: |
Initial Conditions were 2% B. The %B was increased to 52.8% over 6 minutes and then returned to 2% over 0.5 min. The system was allowed to re-equilibrate for 1.5 min. The total cycle time was 8.0 min. |
|
MS System: |
XEVO TQD Mass Spectrometer |
|
Ionization mode: |
ESI Positive |
|
Acquisition mode: |
MRM (See Table 1 for transitions) |
|
Capillary voltage: |
1 kV |
|
Collision energy (eV): |
Optimized for individual compounds (See Table 1) |
|
Cone voltage (V): |
Optimized for individual compounds (See Table 1) |
|
Data management: |
All data was acquired and analyzed using Waters MassLynx Software v.4.1 |
All compounds and internal standards (IS) were purchased from Cerilliant (Round Rock, TX). Complementary, deuterated internal standards were used for all compounds with the exception of hydromorphone-3-glucuronide, codeine-6-glucuronide, norbuprenorphine-glucuronide, norfentanyl, and buprenorphine-glucuronide. For these compounds, a deuterated IS with the most similar response was chosen as a surrogate.
A combined stock solution of all compounds (10 µg/mL; 2.5 µg/mL for fentanyl and norfentanyl) was prepared in methanol. Working solutions were prepared daily by preparing high standards and QCs in matrix (oral fluid) and performing serial dilutions to achieve the desired concentrations. Calibrator concentrations ranged from 5-500 ng/mL for all analytes with the exception of fentanyl and norfentanyl, which were prepared at 25% of the concentration of the other analytes (1.25-125 ng/mL). A combined internal standard stock solution (5 µg/mL; 1.25 µg/mL for fentanyl and norfentanyl) was prepared in methanol. Working IS solutions were prepared daily in MeOH at 500 ng/mL.
Oral fluid samples were collected with the Quantisal collection device from Immunalysis according to the manufacturer’s directions. The collection applicator was saturated with oral fluid, and then placed in the collection vial, which contained 3.0 mL of sample stabilization buffer. This was claimed to be the equivalent of collecting 1.0 mL ± 0.1 mL of sample. The collection kit was stored overnight to simulate the transit time of the sample and to allow for complete equilibration between the sample in the applicator and the stabilization buffer in the collection vial.
400 µL aliquots of buffer stabilized oral fluid samples (equivalent to 100 µL oral fluid) were pretreated by adding 200 µL 4% H3PO4 and 20 µL of the working IS mixture (500 ng/mL in MeOH). Wells in the 96-well Oasis MCX μElution plate (p/n 186001830BA) were conditioned with 200 µL MeOH followed by 200 µL MilliQ water. The entire pretreated sample was then added to each well. After loading, the wells were washed with 200 µL of 2% formic acid, followed by 200 µL of methanol and 200 µL of isopropanol (IPA). All samples were then eluted with 2 x 50 µL of 60:40 ACN:IPA containing 5% of a concentrated NH4OH solution (Fisher, 20-22%). After elution, all samples were evaporated under N2 to dryness at 37 °C (approximately 5 min.) and reconstituted with a solution of 98:2 water: ACN containing 0.1% formic acid and 0.1% (by volume) human plasma. 10 µL was injected onto the LC-MS/MS system.
Recovery was calculated according to the following equation:
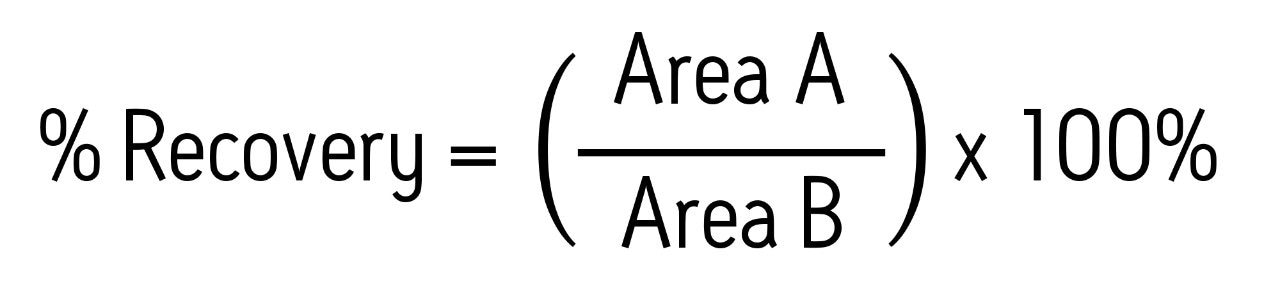
Area A refers to the peak area of a sample spiked with analytes before extraction, and area B refers to the peak area of a sample in which the analytes were spiked into the final eluate after extraction.
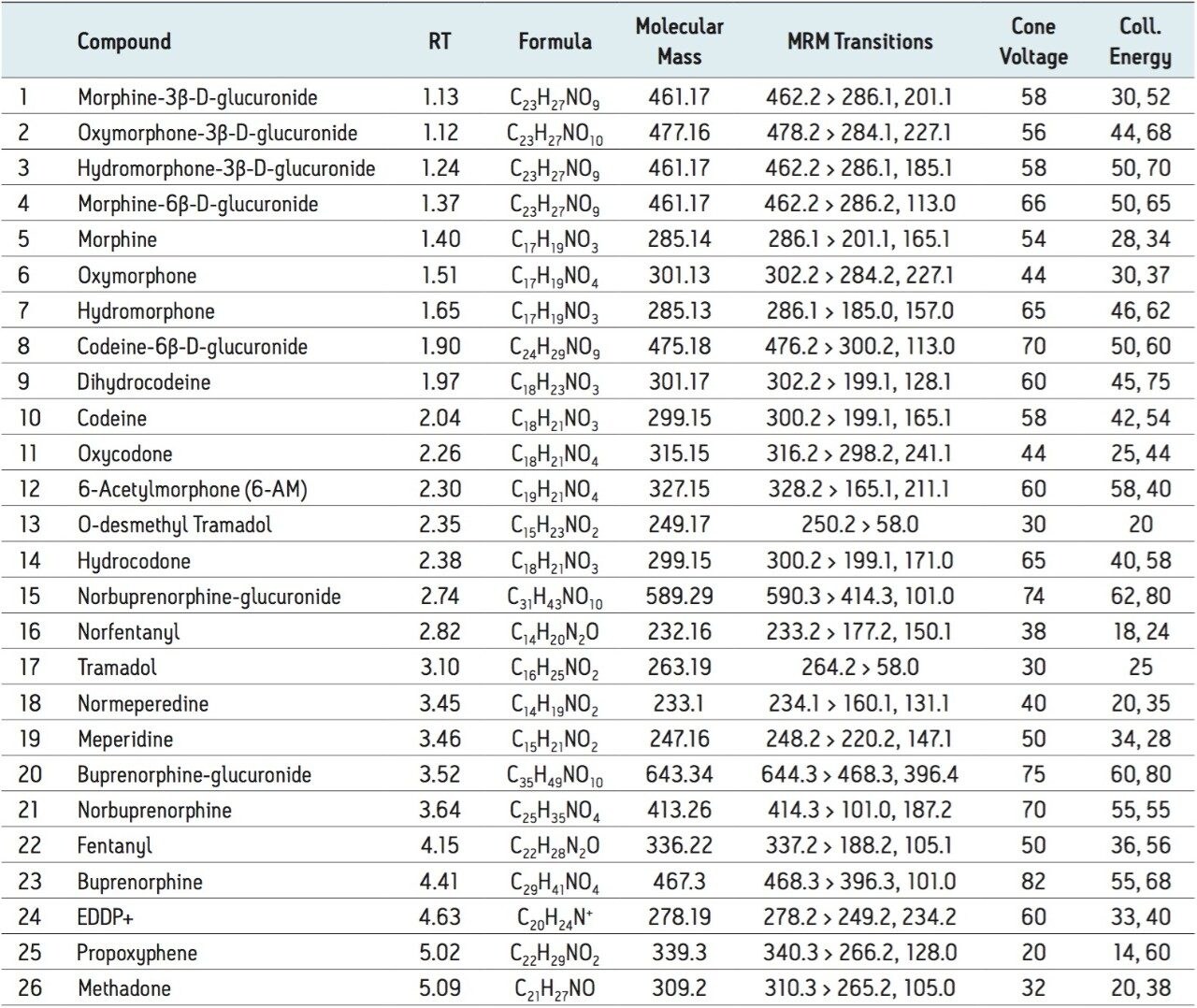
The 26 compounds and metabolites screened are listed in Table 1 and constitute a comprehensive panel of natural opiate drugs, semi-synthetic opioids, and synthetic narcotic analgesic compounds. Most of the compounds are weak bases, with pKa values of approximately 8-9. They have a wide range of polarities, with LogP values ranging from -3.48 for morphine-3β-d-glucuronide to 5.0 for methadone. MRM transitions, cone voltage and collision energies are also listed in Table 1.
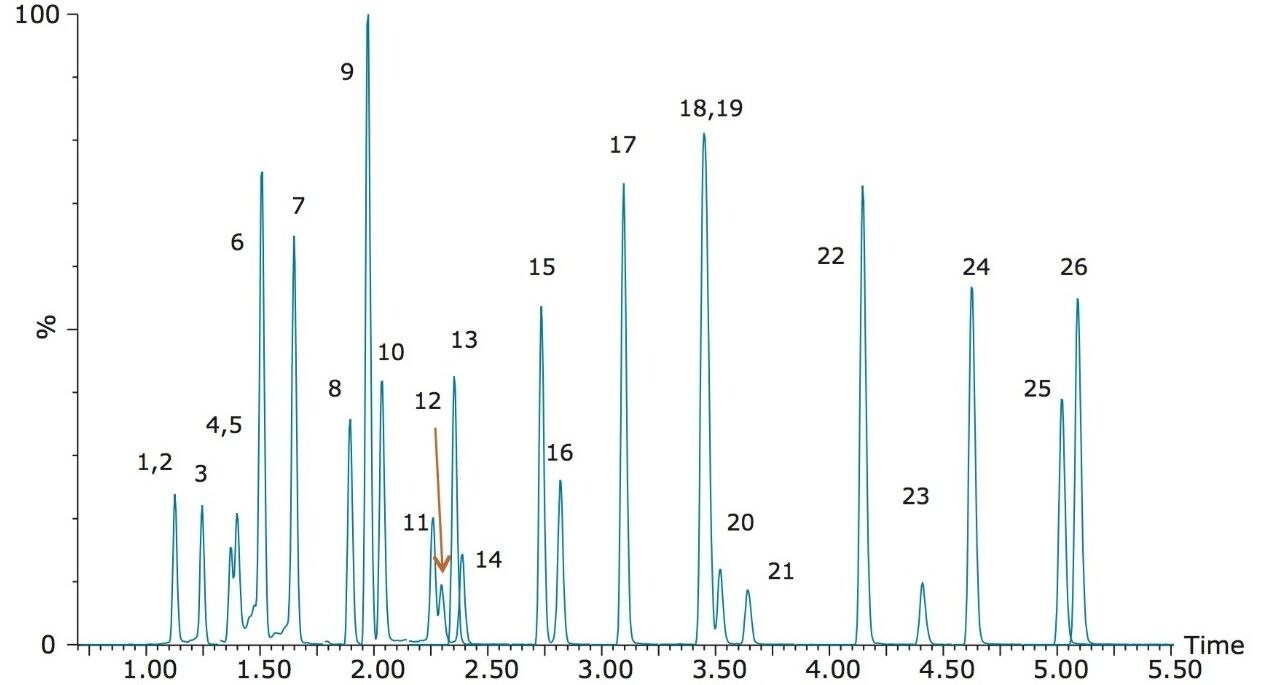
A representative chromatogram of all compounds is shown in Figure 1. Peak assignments can be found in Table 1. Using an ACQUITY UPLC BEH C18 Column (1.7 μm, 2.1 x 100 mm), we were able to analyze all compounds in under 5.5 minutes with baseline separation between all critical pairs of isomers, such as morphine-3-glucuronide, morphine-6-glucuronide and hydromorphone-3-glucuronide (compounds 1, 3, and 4, respectively).
Recovery was evaluated using both IPA and MeOH as a co-elution solvent with ACN. Both solvents resulted in similar recovery patterns for the 26 opiate compounds. When MeOH was used, recoveries were slightly better for the 4 earliest eluting glucuronide metabolites. However, the average recovery for all compounds was improved when using IPA. Eluting with 60:40 ACN:IPA resulted in an average recovery of 78.8% for all compounds vs. 74.2% using 60:40 ACN:MeOH. Figure 2 shows the average recovery for all compounds when eluted with 60:40 ACN:IPA.
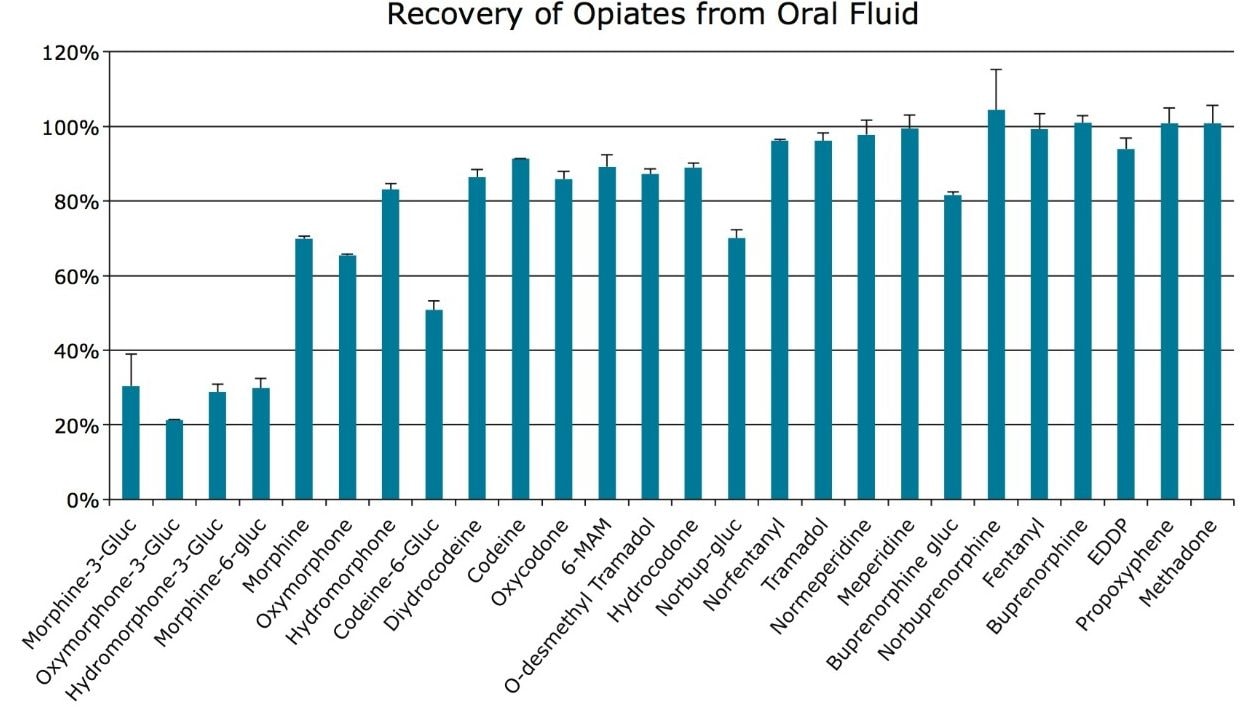
For this application, evaporation of the organic eluate and reconstitution in a high aqueous solution (2% ACN) was necessary to prevent strong solvent effects that would otherwise affect the chromatography of the glucuronide metabolites by causing peak distortion that prevents proper retention and integration of the resulting peaks. However, use of the Oasis MCX Plate in the μElution Plate format results in only 100 μL of eluate that is easily evaporated in under 5 minutes. An additional benefit of using the μElution plate format is that only 100 μL of sample is needed for the assay. This can be a significant advantage for oral fluid analysis, since sample sizes are often quite small (1.0 mL). The ability to use minimal sample volumes allows for repeat analysis, or the use of additional aliquots for other analyses, if necessary.
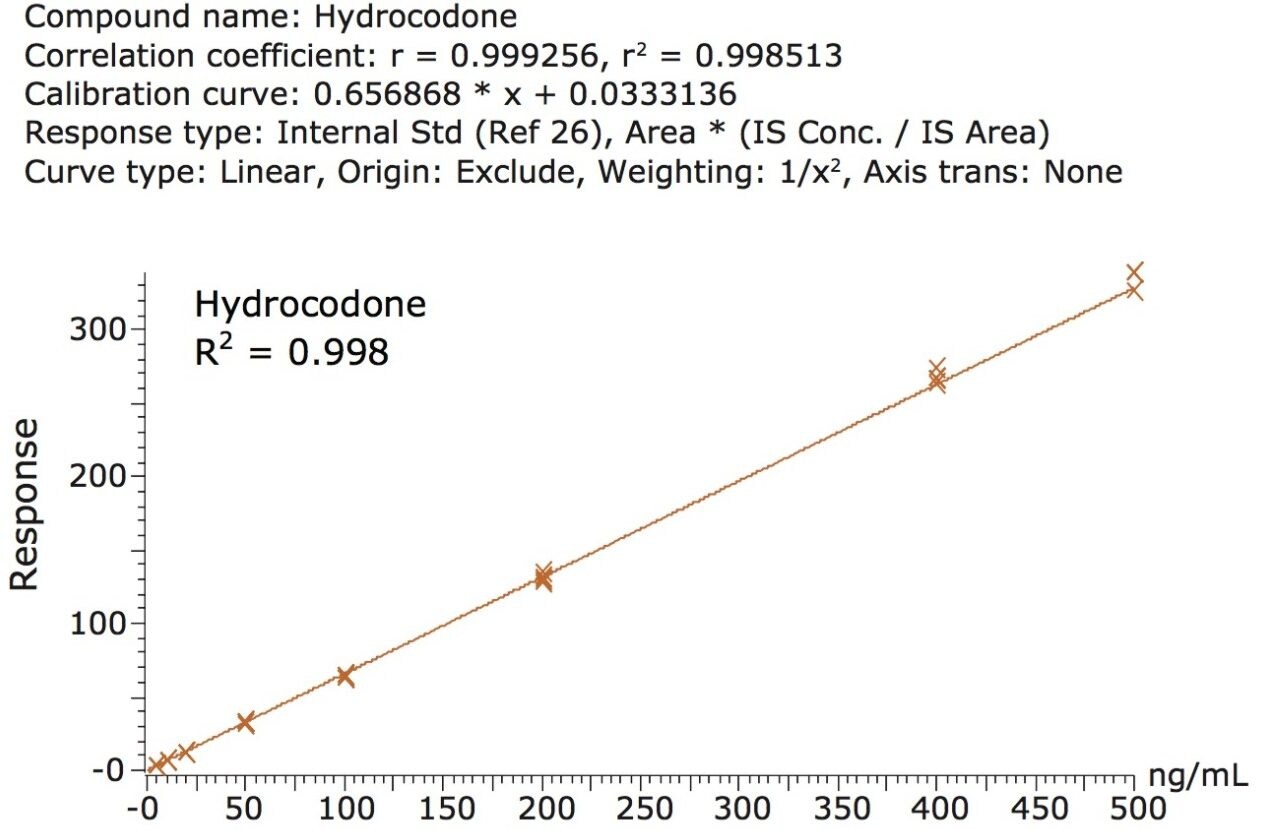
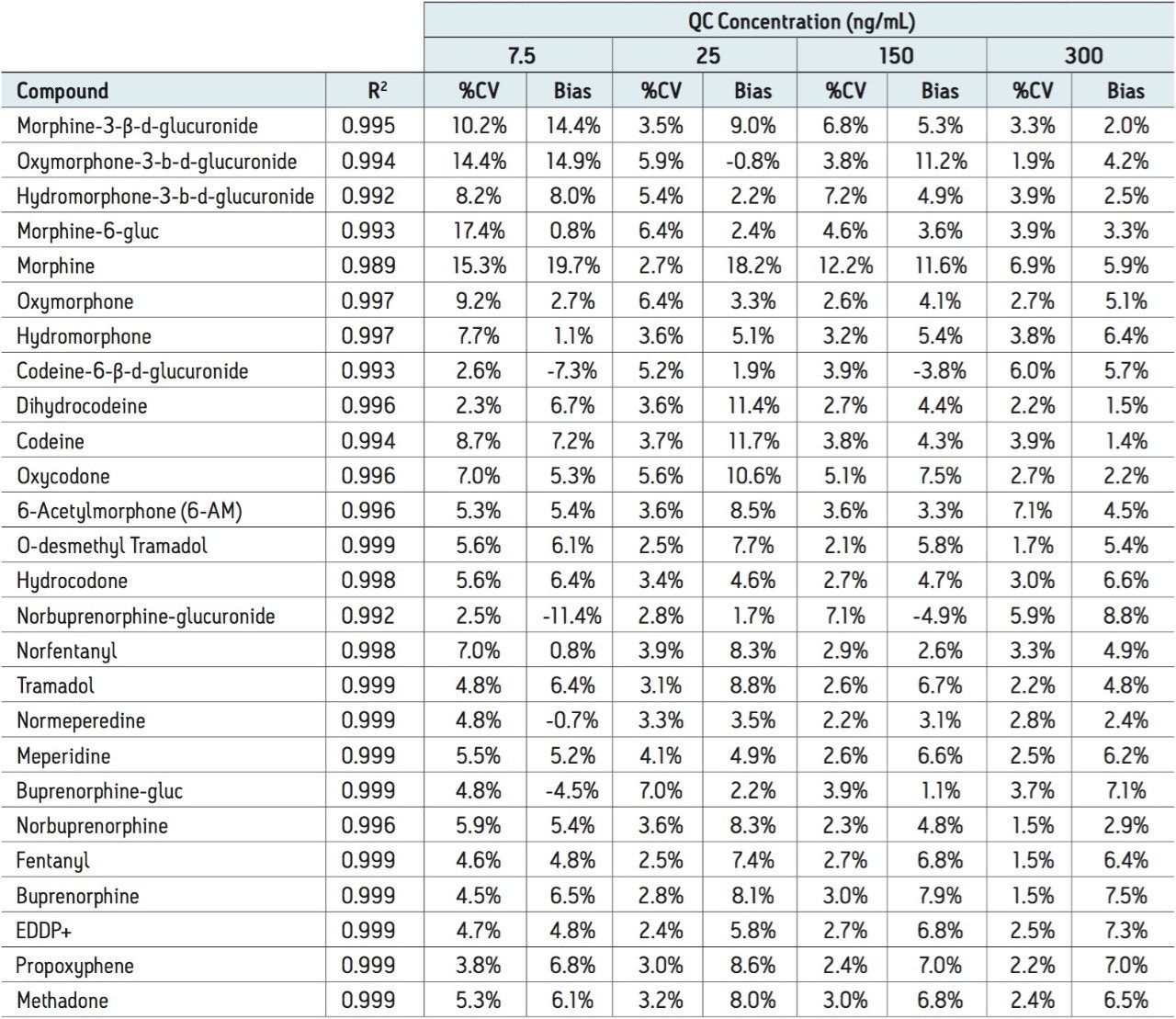
Calibration and quality control (QC) results indicate that this method is linear, accurate and precise. Calibration standards were prepared in oral fluid at concentrations ranging from 5-500 ng/mL (1.25-125 ng/mL for fentanyl and norfentanyl). An example calibration curve is shown for hydrocodone in Figure 3. The mean accuracies and R2 values for the calibration curves are listed in Table 2. All compounds had R2 values of at least 0.989 and many were 0.995 or greater. Quality control samples (N=4) were prepared at 4 concentrations: 7.5, 25, 150, and 300 ng/mL. Analytical accuracy and precision were very good. With only 2 exceptions, all QC results were within 15% of their intended values and all but 2 points had % CVs that were under 15%.
The method presented here demonstrates the advantages of mixed-mode µElution SPE combined with UPLC-MS/MS for the analysis of 26 opioid compounds and metabolites of interest. All compounds are analyzed in under 5.5 minutes with complete resolution of all isobaric compound pairs. Linearity, analytical accuracy, and precision were excellent over the entire calibration range for all 26 compounds. The μElution format enabled the extraction of 100 μL aliquots of oral fluid, leaving the remaining sample for additional assays, or repeat analyses, if necessary. The ability to achieve LOQs of 5 ng/mL for nearly all analytes and the ability to measure glucuronide metabolites directly without hydrolysis make this method well suited for the analysis of these compounds in oral fluid.
720004838, June 2014