For forensic toxicology use only.
This application note uses a panel of natural and synthetic opioid drugs to demonstrate the use of a systematic screening strategy for UPC2 method development.
UltraPerformance Convergence Chromatography (UPC2) is a novel technology that applies the performance advantages of UPLC to supercritical fluid chromatography (SFC). Combining the use of CO2, a renewable “green” solvent with a high diffusion constant, and multiple complementary stationary phases, UPC2 represents an analysis technique that is orthogonal to UPLC and can be used to solve many troublesome separations that challenge conventional LC or GC analyses. Due to the lack of familiarity that many analytical chemists may have with UPC2, it is essential to develop straightforward strategies for method development and optimization. In order to demonstrate the process of chromatographic method development for UPC2, this technique was applied to the analysis of a panel of natural and synthetic opioid drugs. These compounds represent an important category of drugs in clinical medicine. Applications include workplace drug testing, pain management monitoring, and compliance with drug treatment programs. Other than one report published nearly 25 years ago,1 we were unable to find any applications of SFC to the analysis of opioid drugs. Thus, we decided to evaluate the utility of UPC2 for the analysis of this important class of compounds.
This application note highlights a systematic strategy for method development using four different commercially available stationary phases combined with four different organic co-solvent additives that allows for the quick determination of optimal starting conditions for method development. Following the initial screening protocol, final method optimization was straightforward and rapid, resulting in a method that analyzes 19 natural, semi-synthetic, and synthetic opioids and related drugs used for pain management, addiction treatment, and drug abuse monitoring. The resulting method enables the analysis of all compounds in less than two minutes with acceptable peak shapes and a total cycle time of four minutes.
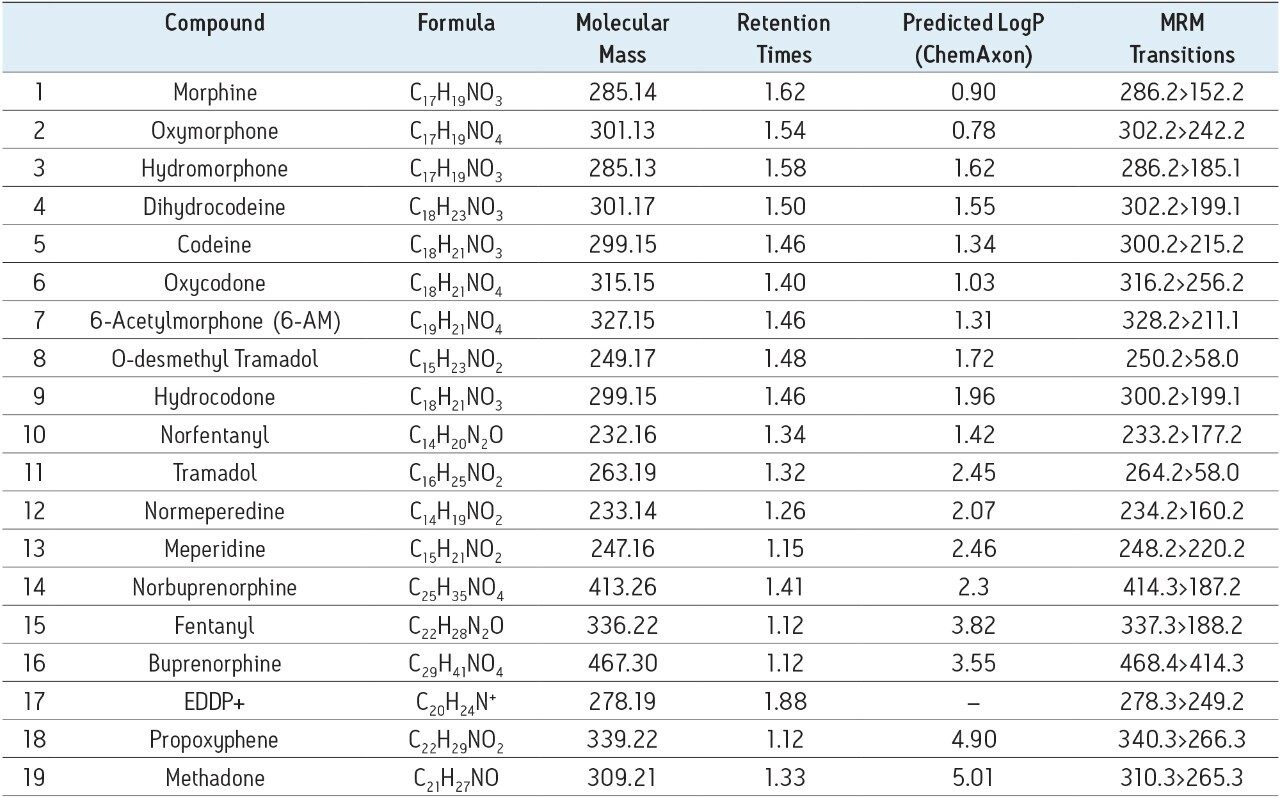
The 19 compounds screened, listed in Table 1, constitute a comprehensive panel of natural opiate drugs, semi-synthetic opioids, and synthetic narcotic analgesic compounds used for pain management. Most all of the drugs are weak bases, with pKa values between approximately 8 and 9. They have a wide range of polarities, with LogP values ranging from 0.78 for oxymorphone to 5.0 for methadone, as shown in Table 1. MRM transitions used are also listed in Table 1. Stock solutions of all compounds were prepared in methanol, and working solutions were dissolved in either methanol, isopropanol (IPA), or a combination of 60:40 acetonitrile/methanol. The concentration of all compounds in the working solutions was 500 ng/mL.
|
System: |
ACQUITY UPC2 |
|
Column: |
ACQUITY UPC2 BEH, 2.1 x 50 mm, 1.7 μm (p/n 186006558) |
|
Column temp.: |
55 °C |
|
Injection volume: |
2 μL |
|
Flow rate: |
1.5 mL/min |
|
Mobile phase A: |
CO2 |
|
Mobile phase B: |
MeOH with 0.4% formic acid and 40 mM NH4COOH |
|
Vials: |
LC-MS Certified 12 x 32 mm screw neck Maximum Recovery (p/n 600000749CV) |
|
Gradient: |
Initial conditions were 2% B. The %B was increased to 50% over 2 min and held at 50% for 0.5 min. The %B was then returned to 2% over 0.1 min, and the system was allowed to re-equilibrate for 1.4 min. The entire cycle time was 4.0 min. |
|
Mass spectrometer: |
ACQUITY TQD |
|
Make up flow: |
1% formic acid in methanol (0.25 mL/min) |
|
Ionization mode: |
ESI positive |
|
Acquisition mode: |
MRM (See Table 1 for transitions) |
|
Capillary voltage: |
1 kV |
|
Collision energy: |
Optimized for individual compounds |
|
Cone voltage: |
Optimized for individual compounds |
|
Data management: |
MassLynx Software |
In order to determine the optimal starting conditions for the analysis of opioid drugs using UPC2, a series of screening runs was performed using a four-position column manager to evaluate four different column chemistries and four different mobile phase additives. The columns used were Waters UPC2 BEH, BEH 2-EP, CSH Fluoro-Phenyl, and HSS C18 SB. Methanol was used for the B mobile phase with the following additives: None (MeOH only), 0.2% formic acid, 0.2% NH4OH, or 0.2% formic acid + 20 mM NH4COOH. The initial screening gradient started at 5% B and increased to 75% B over 4 min. The flow was returned to 5% B over 1 min, and held at the initial conditions for 1.4 min to re-equilibrate the column. Flow rates for each column were set to keep the back pressure below the system limit of 6000 psi and were 1.5 mL/min for the BEH and 2-EP columns and 1.0 mL/min for the HSS and PFP CSH Fluoro-Phenyl columns. Initial screenings were performed with a limited group of compounds including fentanyl, morphine, oxymorphone, oxycodone, and methadone. These represent a range of polarities chosen to simplify the initial screening process.
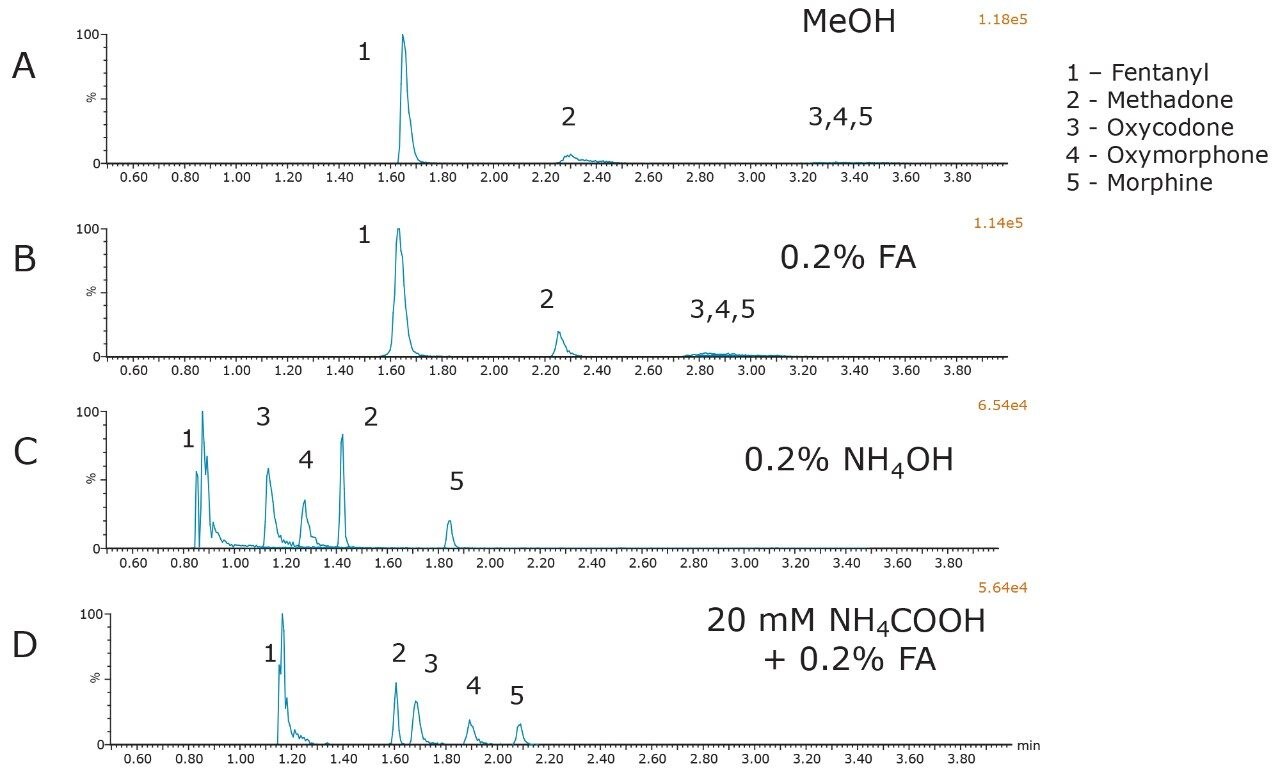
The initial evaluation of MPB additives on the BEH column is shown in Figure 1. All compounds were at a concentration of 500 ng/mL. It is quite apparent from this figure that both pure methanol and the addition of 0.2% formic acid produced broad peak shapes with low intensity in MS for oxycodone, oxymorphone, and morphine. In contrast, the addition of either 0.2% NH4OH or 0.2% formic acid + 20 mM NH4COOH resulted in acceptable peaks for most of the compounds used in this initial screen. This is consistent with previous reports of alkaline conditions being used to achieve good chromatographic performance for bases in general, and opiates in particular under SFC conditions.1,2 Closer evaluation of the bottom two chromatograms revealed that the buffered additive resulted in greater retention and improved peak shape compared to the addition of concentrated ammonia only
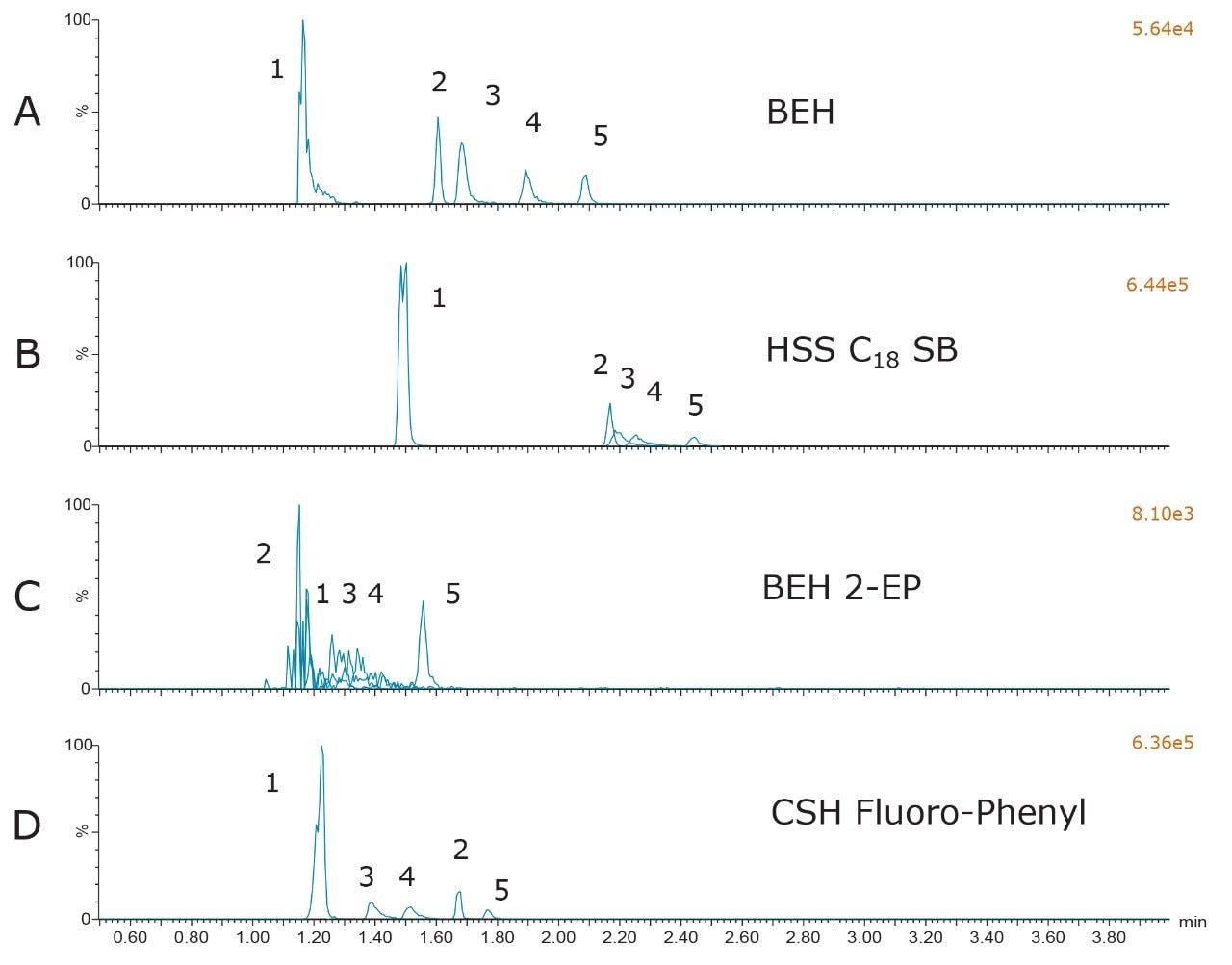
The results from the screening of the different columns with the chosen mobile-phase additive used in Figure 1 are shown in Figure 2. Although the data is not shown, as with the BEH particle, all of the columns performed poorly when unmodified methanol or methanol containing formic acid was used as MPB. In addition, for all columns, the behavior was similar to the BEH column in that, while 0.2% ammonium hydroxide resulted in improved peak shapes when compared to methanol or methanol with 0.2% formic acid, the best performance relative to peak shape, retention, separation, and sensitivity was achieved with the combination of formic acid and ammonium formate in MPB. Figure 2 shows the chromatography on all four columns using the buffered mobile-phase additive used in Figure 1D. The 2-EP particle did not result in acceptable peak shapes for any of the compounds, with the exception of morphine. The HSS C18 SB column resulted in acceptable peaks for all of the compounds tested; however, the peak resolution was inferior to that of the BEH or CSH Fluoro-Phenyl columns. The use of the CSH Fluoro-Phenyl column resulted in baseline resolution of all peaks, and slightly different selectivity than the BEH particle. Based upon these results, it was decided to continue chromatographic optimization using both the BEH and CSH Fluoro-Phenyl columns.
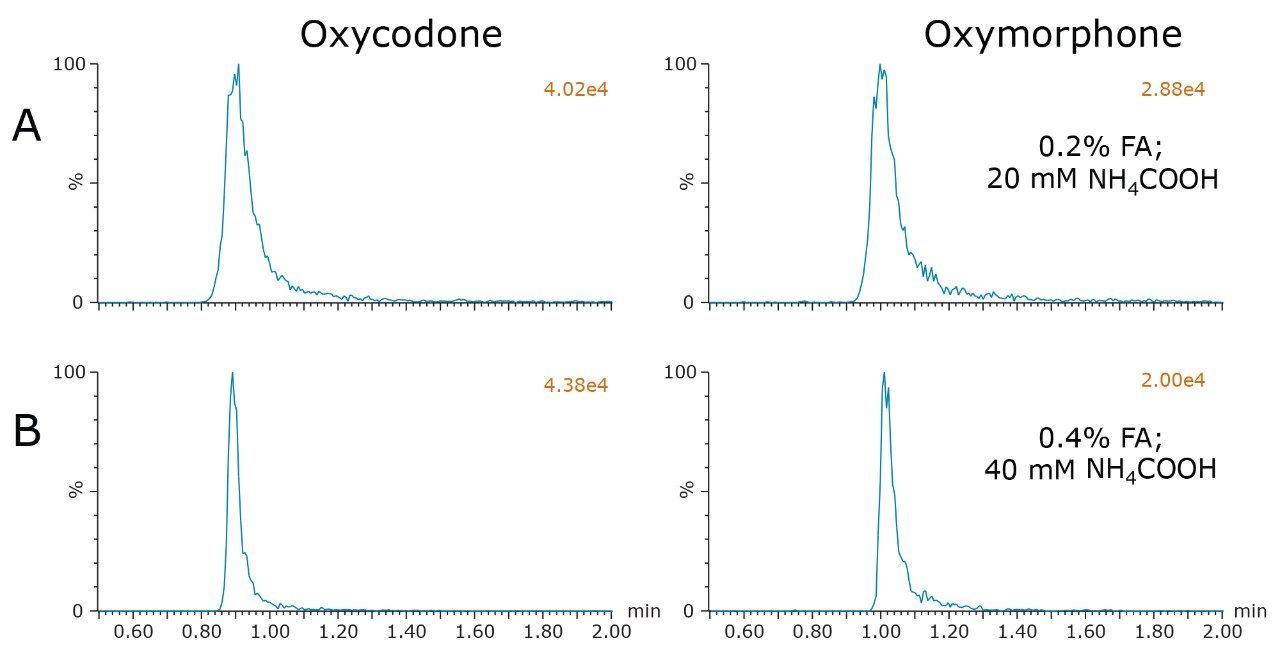
Analysis of the remaining compounds using the chosen conditions confirmed the selection of a buffered additive (formic acid + NH4COOH), as well as the choice of either the BEH column or the CSH Fluoro-Phenyl column. With very few exceptions, all compounds demonstrated excellent performance under these conditions. Predictably, however, there were a few compounds in the extended mix that required additional optimization. One pair of compounds included oxymorphone and oxycodone. These two compounds demonstrated substantial peak tailing and broadening compared to the other analytes. Chromatograms for these two peaks are shown in Figure 3A. Peak widths at 5% peak height were 16.5 and 22.5 seconds for oxycodone and oxymorphone, respectively, compared to a peak width of 4 seconds for morphine. Since retention mechanisms under supercritical conditions are thought to be strongly influenced by interactions between the solutes and the stationary phase,2,4 it was hypothesized that secondary interactions between these compounds and the stationary phases could be contributing to the poor peak shape. In an attempt to minimize any possible secondary interactions, the concentrations of the additives in MPB were doubled to 0.4% formic acid and 40 mM NH4COOH. The resulting chromatograms, shown in Figure 3B, reveal that this change did indeed reduce the peak widths for these two compounds. Peak widths for oxycodone and oxymorphone were reduced to 7.2 and 9.9 seconds, respectively, a reduction of over 50% from the original conditions. Equally important, the chromatographic performance of the other compounds was not adversely affected.
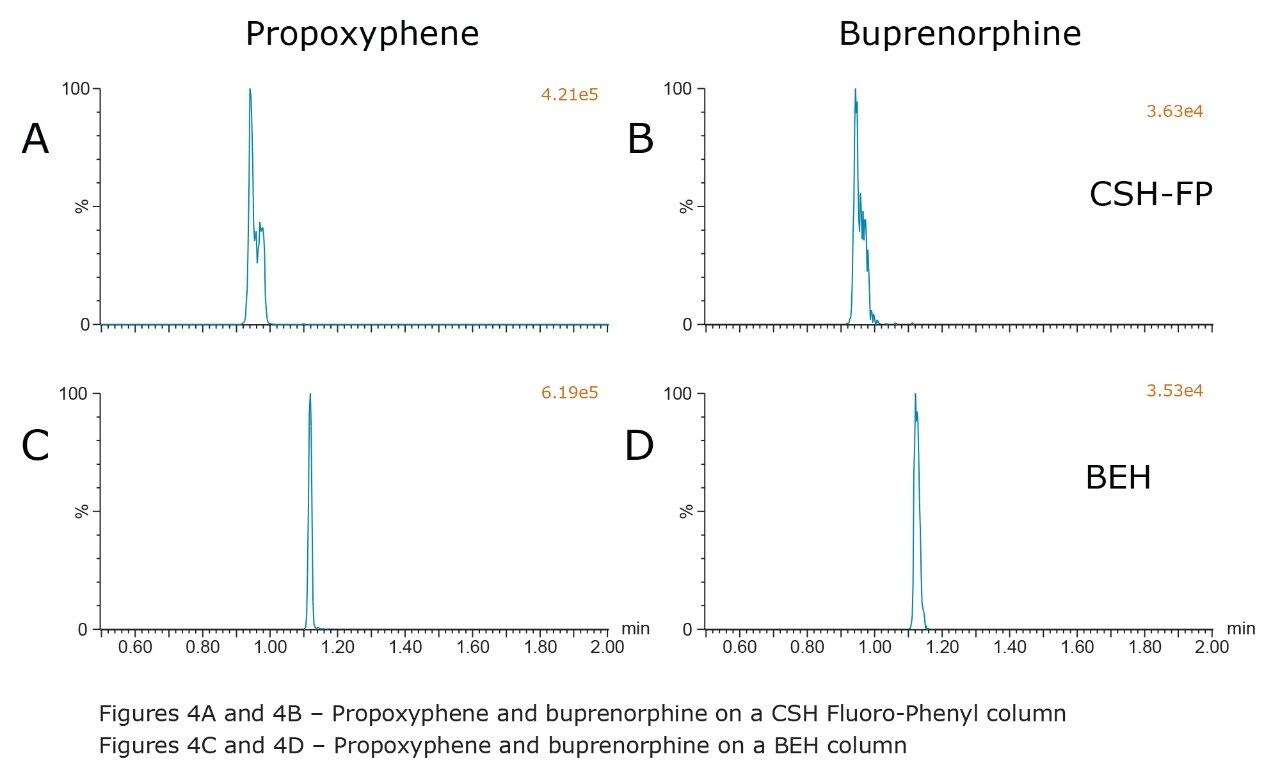
Another set of compounds that presented chromatographic challenges were many of the early eluting, less polar compounds such as meperidine, fentanyl, methadone, propoxyphene, and buprenorphine. Based upon the initial screening data, both the CSH Fluoro-Phenyl and BEH columns showed promising results for these compounds; however, split peaks and poor chromatography were observed on the CSH Fluoro-Phenyl column using the final gradient conditions described in the Experimental section, as shown in Figures 4A and 4B. In contrast, using the BEH column resulted in acceptable peak shapes for all of these compounds, possibly due to the increased retention or reduced solvent effects resulting with this column. Figure 4 shows the performance of propoxyphene and buprenorphine on both the CSH Fluoro-Phenyl and BEH columns using the final conditions where the gradient starts at 2% MPB. These figures clearly show the improvement in peak shape when using the BEH column, possibly because of the increase in retention with this column
The final parameter evaluated was the choice of sample diluent. One of the advantages of using UPC2 for bioanalysis is the compatibility with solvents used for sample preparation. Whether selecting solid-phase extraction, liquid-liquid extraction, or protein precipitation, the final extract is often dissolved in an organic solution that may not be compatible with reversed-phase conditions without dilution. UPC2, however, is compatible with many organic solvents, eliminating the requirement for evaporation and reconstitution typically needed in reversed-phase systems. During the course of these experiments, sample diluents of methanol, IPA, and a combination of 60:40 acetonitrile/methanol that is often used to elute samples when using Waters’ Oasis μElution Plates were evaluated. No examples of solvent effects when comparing the diluents of IPA and 60:40 ACN/MeOH were observed. When methanol alone was used as the sample diluent, there were some adverse chromatographic effects such as peak broadening and splitting seen in early eluting compounds such as fentanyl.
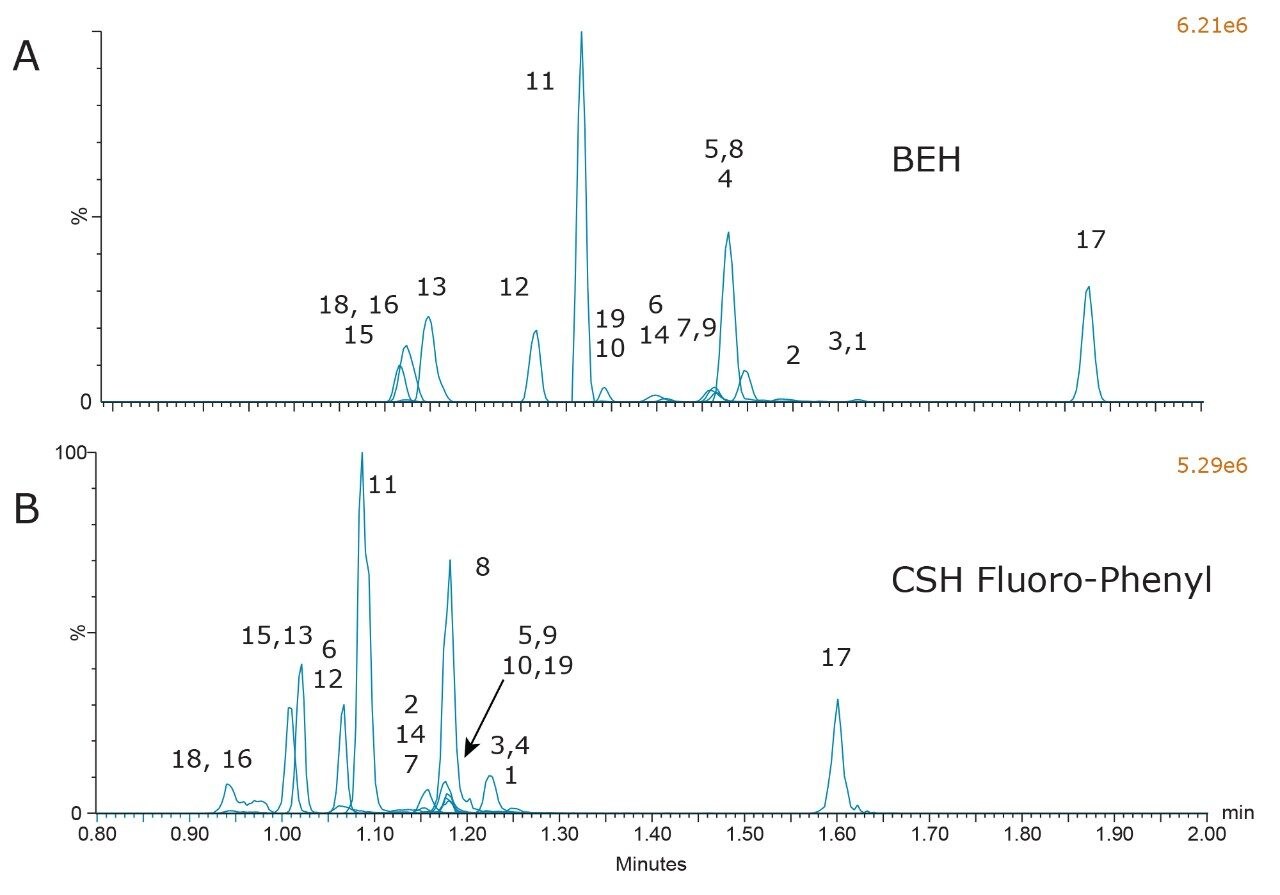
Combined chromatograms of the final method conditions are shown in Figure 5. Compound identifications and retention times are listed in Table 1. Panel A shows the results from the BEH column and panel B shows the results from the CSH Fluoro-Phenyl column. The greater retention of all compounds on the BEH column, and the improved peak shape for propoxyphene and buprenorphine (compounds 18 and 16) are clearly evident. The method still detects all compounds in less than 2 minutes and could be used as a rapid screening method for this class of compounds.
This application note uses a panel of natural and synthetic opioid drugs to demonstrate the use of a systematic screening strategy for UPC2 method development. The simultaneous evaluation of multiple column chemistries and several different mobile-phase additives allows rapid determination of preferred starting conditions for further optimization. This enabled easy and rapid optimization of conditions to achieve a method capable of analyzing 19 different opioid drugs in less than 2 minutes, with acceptable retention and peak shape for all compounds. In addition, this method further highlights the potential utility of UPC2 for the analysis of a wide variety of compound classes.
720004608, March 2013