The objective of this application note is to demonstrate the efficiency and robustness of Auto•Blend Plus Technology for optimization of an IEX method for charge variant separations. A chimeric monoclonal antibody, infliximab, was used as a model therapeutic protein to showcase the application. The Biopharmaceutical Platform Solution with UNIFI was used to perform the analysis, from acquisition to processing and reporting.
Charge variant analysis is critical for characterizing and monitoring quality attributes of therapeutic proteins. Protein modification such as deamidation, N-terminal pyroglutamation, isomerization, sialylated glycans, and C-terminal lysine clipping all contribute to charge variant formation.1 In some cases, such changes affect binding, biological activity, patient safety, and shelf lifetime of therapeutic proteins.
The biopharmaceutical industry relies on tools such as ion exchange chromatography (IEX) and isoelectric focusing (IEF) gel electrophoresis to characterize charge variants. Ion exchange chromatography has been particularly useful in the development of biotherapeutics due to its ease of use, wide applicability, and high resolution.
In-depth characterization of charge heterogeneity of therapeutic proteins from the biopharmaceutical development process requires robust and efficient IEX methods. Method development involves a thorough evaluation of all possible experimental parameters such as buffer/ionic strength, buffer pH, salt gradient, flow rate, and column temperature. However, systematic evaluation on the impact of individual experimental parameters on the separation performance often requires a time-consuming and iterative process that involves preparing and testing discreet buffers of varying composition.
Variation in buffer preparation can lead to inconsistent results, consequently increasing method development time. Waters Auto•Blend Plus Technology takes advantage of the ACQUITY UPLC H-Class System’s quaternary solvent management capabilities, and uses pure solutions and concentrated stocks to address these challenges. Calculation of the percentage of each stock to blend to achieve the desired pH is performed by the Auto•Blend Plus Technology, reducing error, consumable use, and development time.
With such integrated features, the Biopharmaceutical Platform Solution with UNIFI is well suited for robust method development and can be easily automated for increased productivity. The objective of this application note is to demonstrate the efficiency and robustness of Auto•Blend Plus Technology for optimization of an IEX method for charge variant separations. A chimeric monoclonal antibody, infliximab, was used as a model therapeutic protein to showcase the application.
Sample description
A Waters Protein-Pak Hi Res SP, strong cation exchange column (4.6 x 100 mm, 7 μm, P/N 186004930) was conditioned as outlined by the manufacturer. MES monohydrate (P/N AC327761000), MES sodium salt (P/N AC397351000), sodium chloride (P/N S640-500) were purchased from Fisher Scientific. The chimeric mAb evaluated in this study was used as received for all experiments at a concentration of 20 μg/μL.
|
LC system: |
ACQUITY UPLC H-Class with Auto•Blend Plus |
|
Detector: |
ACQUITY UPLC TUV |
|
Absorption wavelength: |
280 nm |
|
Vials: |
Total Recovery vial: 12 x 32 mm glass,screw neck, cap, nonslit |
|
Column: |
Protein-Pak Hi Res SP, 4.6 x 100 mm, 7 μm |
|
Column temp.: |
25 °C |
|
Sample temp.: |
4 °C |
|
Injection vol.: |
3 μL |
|
Flow rate: |
0.5 mL/min |
|
Mobile phase A: |
100 mM MES monohydrate |
|
Mobile phase B: |
100 mM MES sodium salt |
|
Mobile phase C: |
1000 mM NaCl |
|
Mobile phase D: |
18 MΩ H2O |
|
Buffer conditions: |
20 mM MES, pH 6.8 |
|
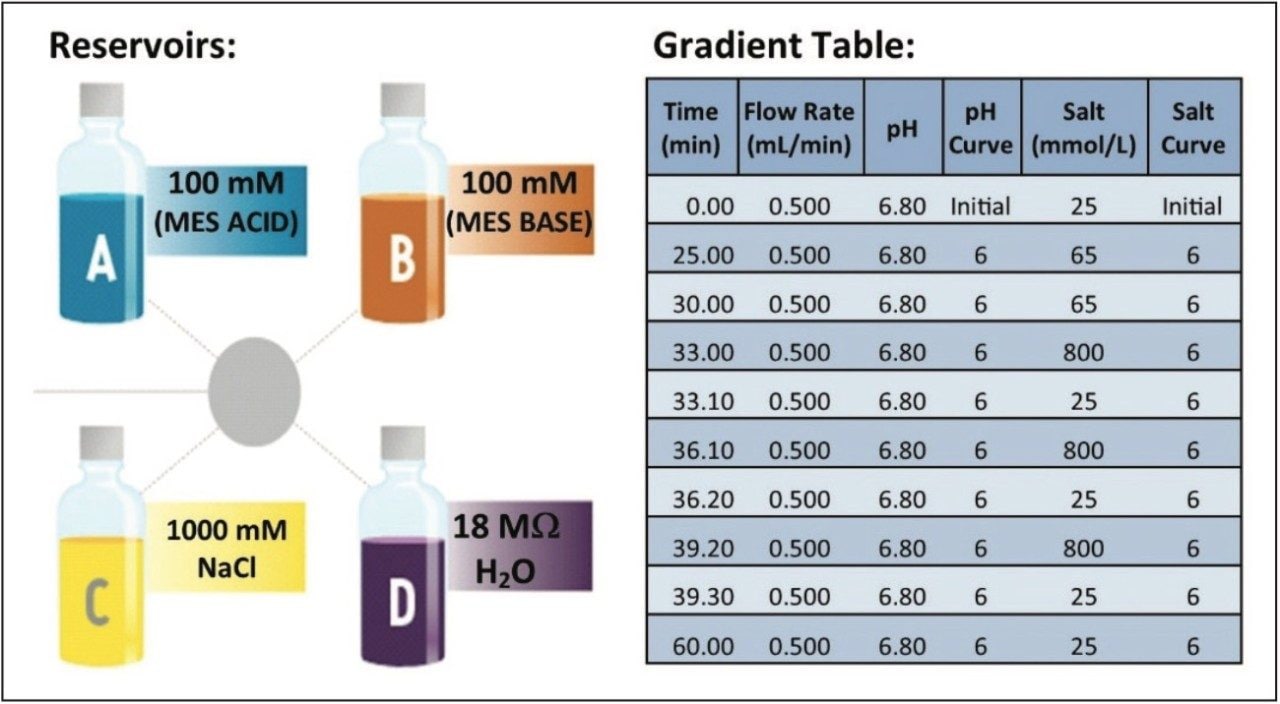
Gradient: |
25 mM to 65 mM NaCl in 25 minutes (see Figure 2) |
UNIFI Scientific Information System, v 1.6
Method development of ion exchange chromatography (IEX) techniques often involves a time-consuming trial and error methodology. The iterative process involves preparing multiple buffers at a specific pH and ionic strength, followed by testing of each buffer system until an adequate separation is achieved. The Auto•Blend Plus Technology system is integrated software that comes standard with an ACQUITY UPLC H-Class System. It is designed to take the guesswork out of method development and increase productivity in the analysis of charge variants. Auto•Blend Plus helps analysts configure the quaternary solvent management system to blend pure solutions and concentrated stocks to achieve a desired gradient (Figure 1). The end user is presented with an easy-to-use gradient table interface, where the gradient is expressed directly in terms of pH and ionic strength. The software automatically calculates the percentage of acid and base required for the specified pH using the known pKa value of the chosen buffer system or an empirical calibration table (Figure 2).
Auto•Blend Plus Technology allows for multiple buffer compositions to be tested from a single set of pure components and can be easily automated to improve productivity.
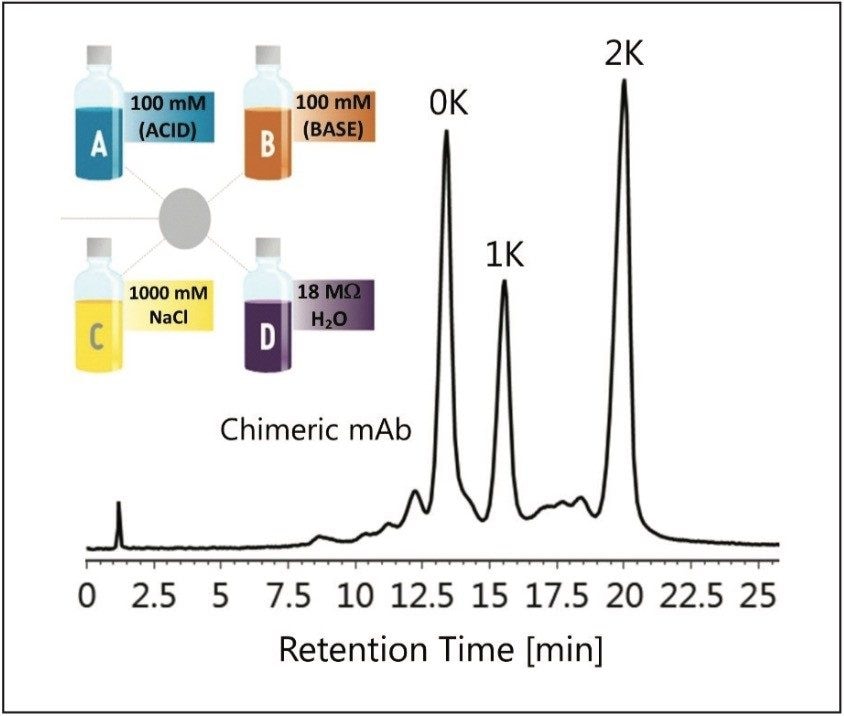
Robustness is a measure of the ability of a separation method to maintain reproducible results with the introduction of small changes in the system. For ion exchange chromatography, these parameters can include pH, protein mass load, and reproducibility. For pharmaceutical companies a robust method can increase productivity with less time spent on method validation. These parameters were explored to evaluate the robustness of method development using the Auto•Blend Plus Technology.
Auto•Blend Plus Technology enables easy system validation and qualification when transferring methods between instruments, analysts, and labs.
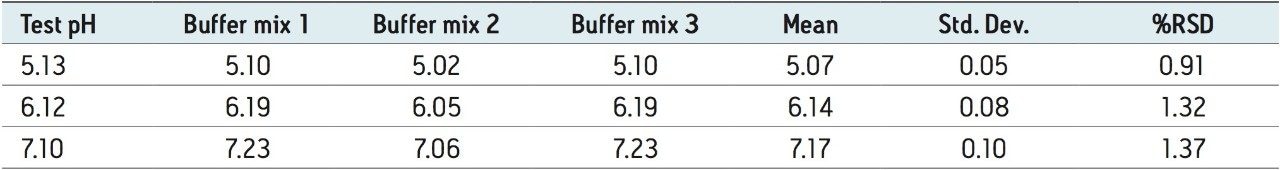
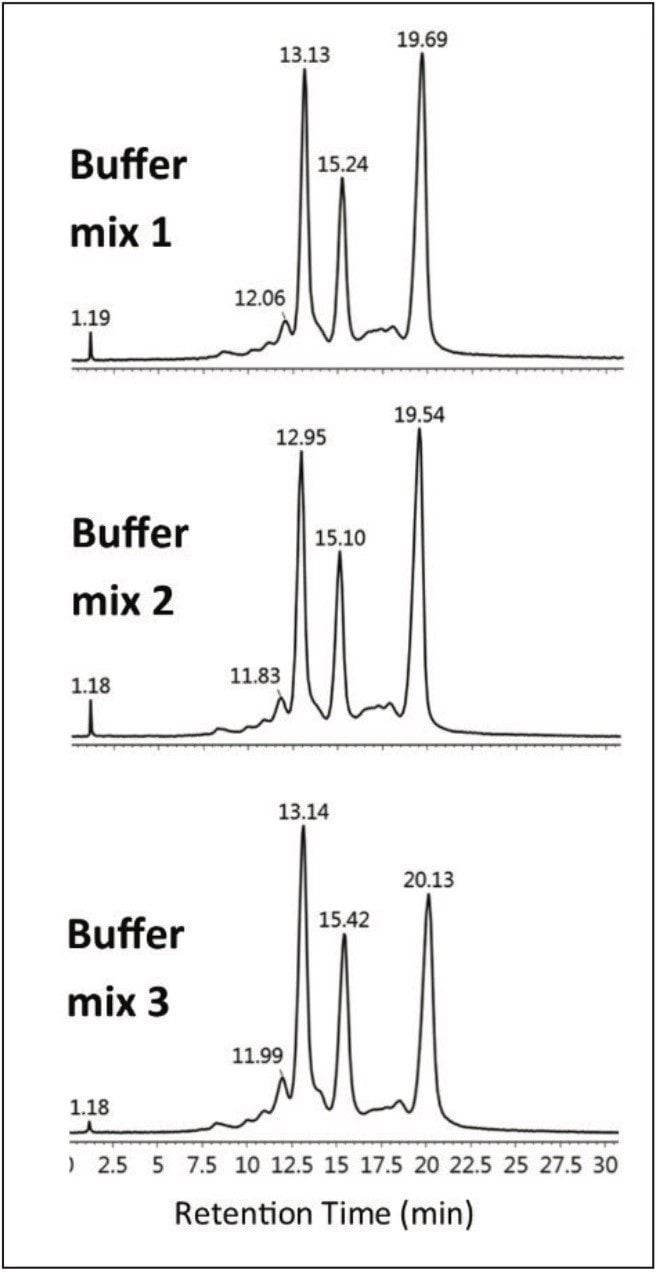
Three separate MES buffer systems were prepared and tested using the outlined protocol, below. From Table 1, it can be readily seen that the experimental pH from each buffer system is in good agreement with the desired test pH. The precision among the three separate buffer systems results in reproducible chromatograms as shown in Figure 3. Auto•Blend Plus Technology can readily be adapted to qualification protocols, minimizing time spent on system validation.
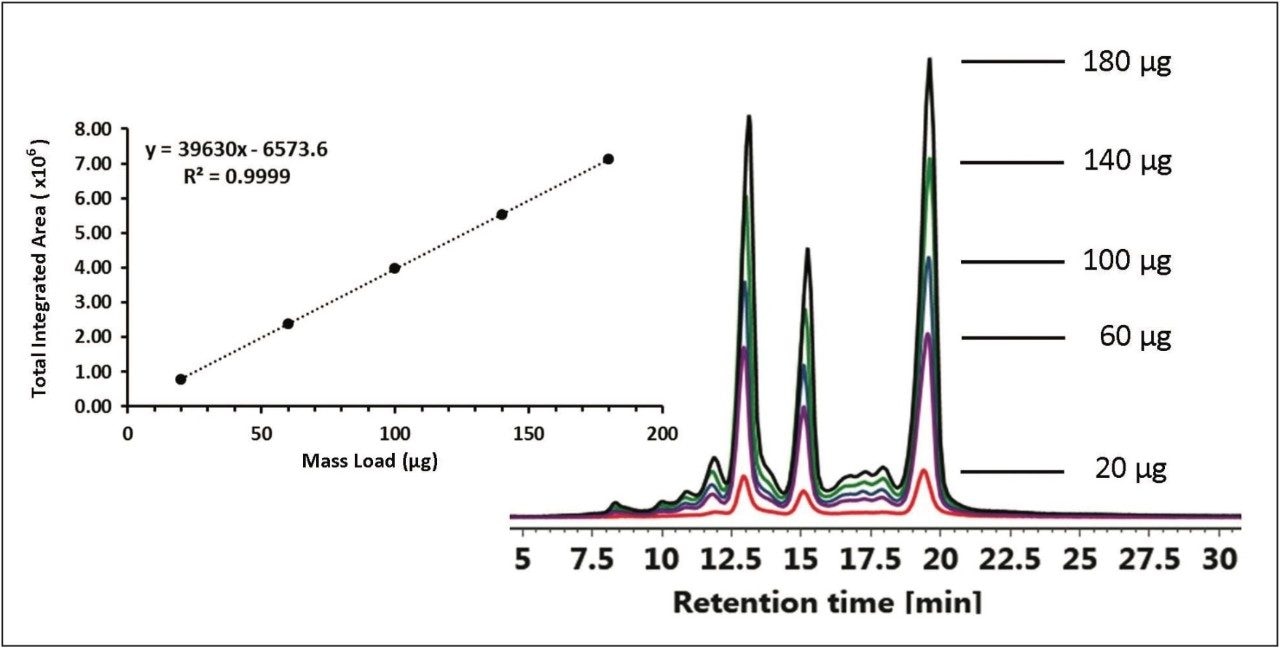
Retention time and column performance can be affected by the amount of protein being injected onto the IEX column.
The effects of protein mass load on column performance were tested by injecting between 1-10 μL of the chimeric mAb stock solution in 1 μL intervals. Total peak area was integrated from 5-30 minutes for each injection. Reproducible retention times were observed over a 9-fold increase in mass load ranging from 20-180 μg of protein as shown in Figure 4. Coupled with the Auto•Blend Plus Technology, the ACQUITY UPLC H-Class System provides a high degree of fidelity for accurate quantification and characterization of charge variants in biotherapeutics.
Automation of analytical techniques can minimizes error in method development as well as increase productivity.
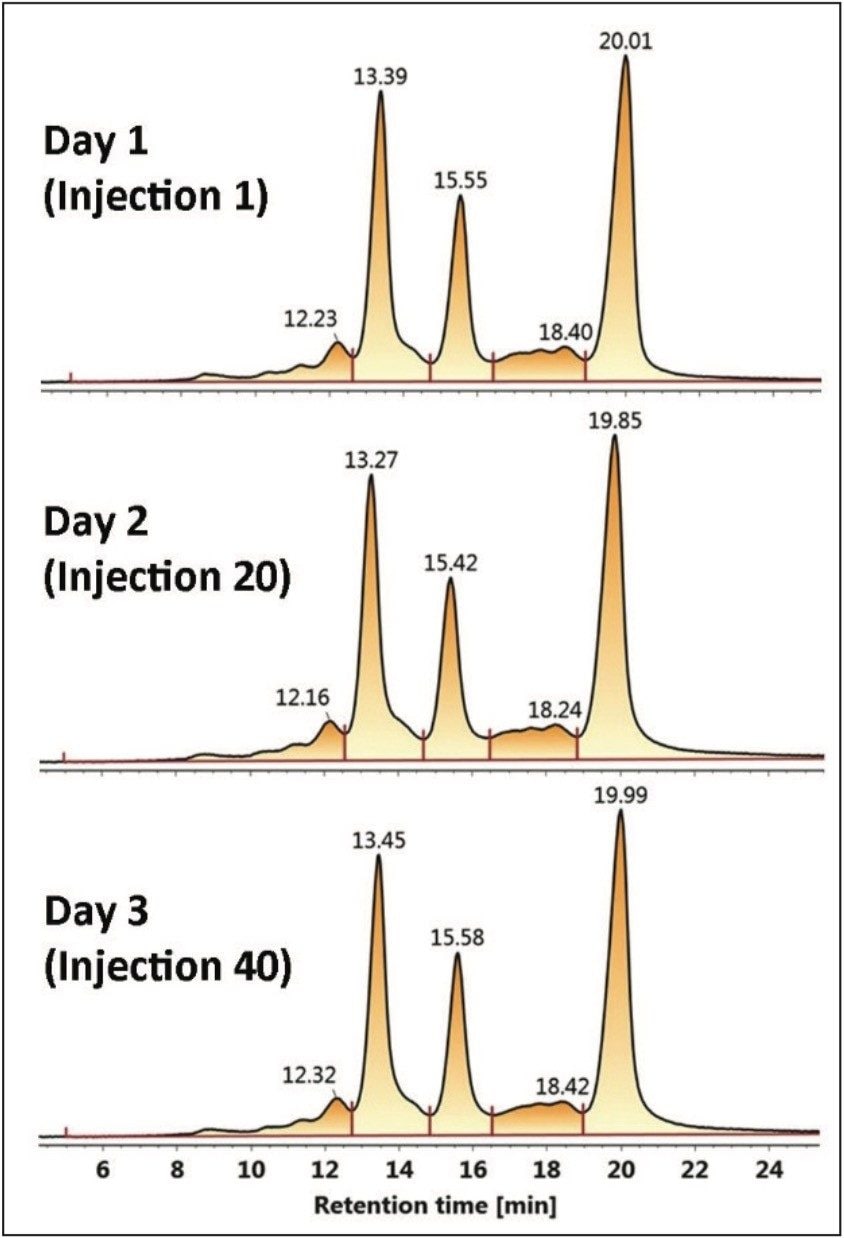
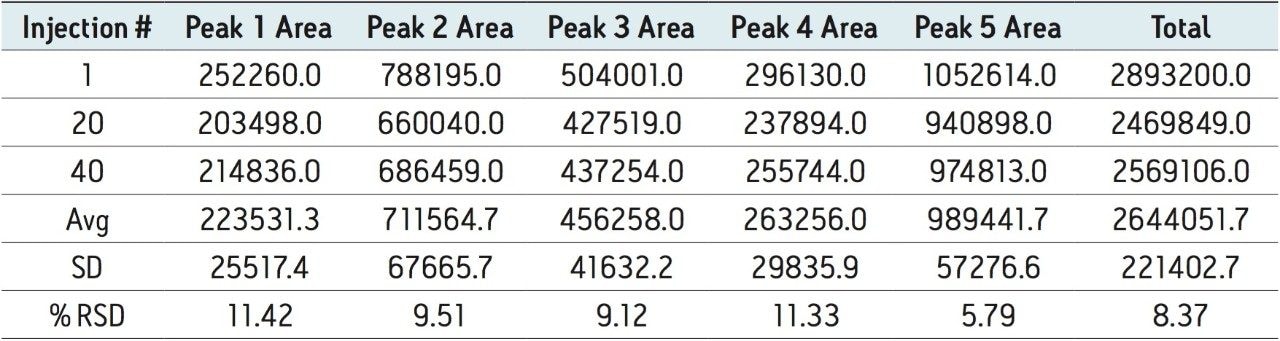
Auto•Blend Plus Technology was evaluated over 40 injections to simulate an unattended analysis over three days. Chromatograms are shown at injectiond 1, 20, and 40. The 60-minute separations as outlined in Figure 2 are comprised of a 30-minute separation gradient and a 30-minute cleaning and reconditioning phase. Integration intervals of five peak areas including the three main C-terminal lysine truncation variants are represented by the vertical drop lines in each chromatogram. Calculated areas of each peak area and total area are listed in Table 2.
It can be seen that Auto•Blend Plus Technology offers reproducible results well within U.S. FDA guidelines2 with covariance of the individual peaks below 12% and the total peak area covariance below 9 %. The ability to automate Auto•Blend Plus Technology, combined with its reproducibility, offers a reliable approach to robust method development for the characterization of charge variants in biotherapeutics.
Analysis of charge heterogeneity profiles that arise during the development process of biopharmaceuticals require robust methods that can be automated, readily deployed, and quickly adapted to meet the demand of fast paced biopharmaceutical industry.
The combination of Auto•Blend Plus Technology with the ACQUITY UPLC H-Class System, which represent a UV-based option of the Biopharmaceutical Platform Solution with UNIFI, simplifies method development by allowing multiple buffer compositions to be tested from a single set of pure components. These features, combined with the ability to automate the process, makes Auto•Blend Plus a powerful tool for robust method development and increasing productivity.
720004847, November 2013