
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the excellent carryover performance of the Alliance HPLC System with target compounds that reveal the different causes of carryover in a chromatographic system.
The flow-through-needle injector design, single wash solvent configuration, and advanced method capabilities deliver outstanding carryover performance, even for analytes that are difficult to eliminate.
For methods requiring quantification, it is important to have a sample delivery design that can accurately and reproducibly deliver sample to the column. One of the contributors to unsatisfactory chromatographic peak area precision can be sample carryover from previous injections. The injector design must, therefore, minimize the susceptibility to carryover. In some instances, severe sample carryover can lead to contamination of the LC system itself which then requires the entire system to be taken off-line for cleaning or even replacement of the contaminated surfaces. An uncomplicated injector design, featuring excellent management of sample carryover, makes it easier for users to optimize the LC method.
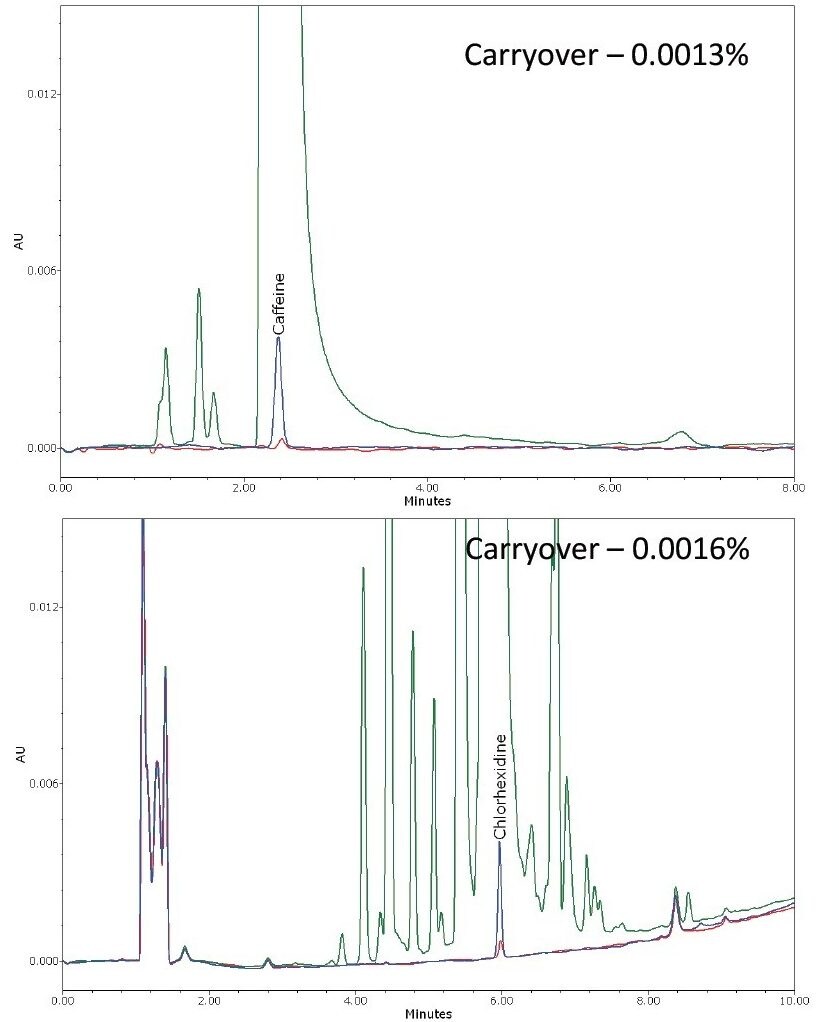
To understand the potential for carryover, LC instrumentation is typically evaluated with two different test compounds. The first is a very polar compound that is highly soluble and demonstrates low surface activity. This class of carryover is volumetric and will affect all components of a sample. This test demonstrates that the injector is sufficiently flushed with solvent during the injector wash cycle to remove the sample, and there is no unswept volume. The second compound is typically ‘sticky’ and known to have surface activity. It is, therefore, much more difficult to remove from the injector surfaces. This class is chemical in nature, sample specific, and affects some sample components more than others. This tests the ability of the wash cycle to manage a wide range of sample types.
The Alliance HPLC System is based on a flow-through-needle design. With this type of injector, the interior of the needle is washed by the mobile phase (gradient) during the analysis. The exterior of the needle is washed in the seal pack, with an appropriate solvent, for a set period of time. The needle wash time can be extended in the method to improve carryover performance. Selection of the needle wash solvent composition is sample-dependent and should easily solubilize the analyte. The Alliance HPLC System supports an extensive range of solvents that may be employed as wash solvents; a critical feature to manage carryover.
The probe compound selected to assess volumetric carryover was caffeine, a small polar molecule that does not tend to adsorb to surfaces. The probe compound used to test the ability to remove a ‘sticky’ analyte was chlorhexidine that can be particularly difficult to remove from LC Systems. Figure 1 shows the carryover observed for the first blank injection using the Alliance HPLC System’s normal wash cycle. The system easily meets the carryover specification for both of these target compounds, with carryover at least 6x lower than the instrument specification.
To further demonstrate the carryover capabilities of the Alliance HPLC System, the advanced wash feature was used. This allows for the needle wash time to be increased to 2x (double) or 4x (extended). By enabling these features, carryover can be reduced even further, as shown in Figure 2.
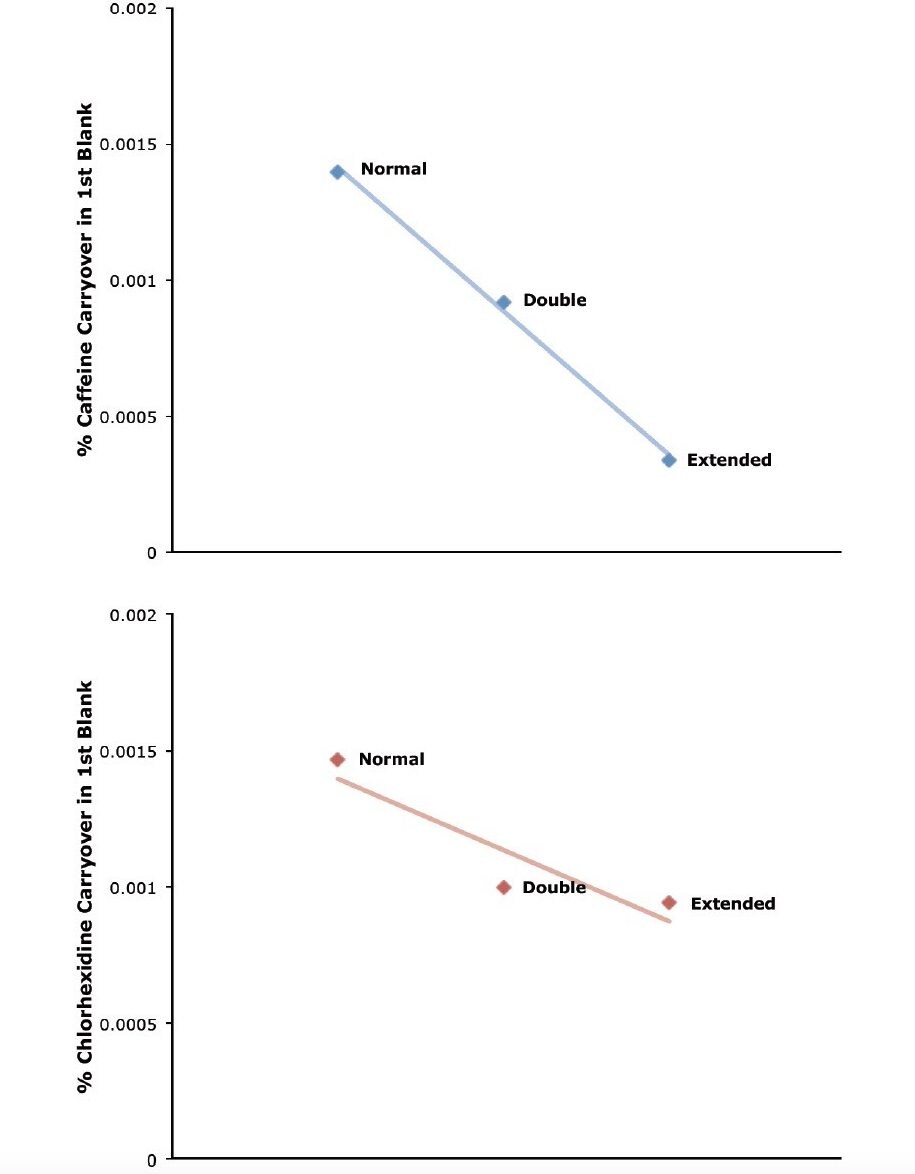
Managing sample carryover can be challenging. This is especially true when deploying methods that require high sensitivity and large dynamic range. The Alliance HPLC System easily manages carryover of both volumetric and chemical adsorption origin for different sample types, providing better quantification for both routine and complex sample analysis.
720004534, December 2012