This is an Application Brief and does not contain a detailed Experimental section.
A compendial normal phase HPLC method for the analysis of anthralin was successfully converted to a supercritical fluid chromatography method using the Waters ACQUITY UPC2 System.
A USP compendial HPLC method was successfully converted to a high quality UPC2 method at a cost of $0.05 per run (compared to $0.90) and was 1.6 times faster.
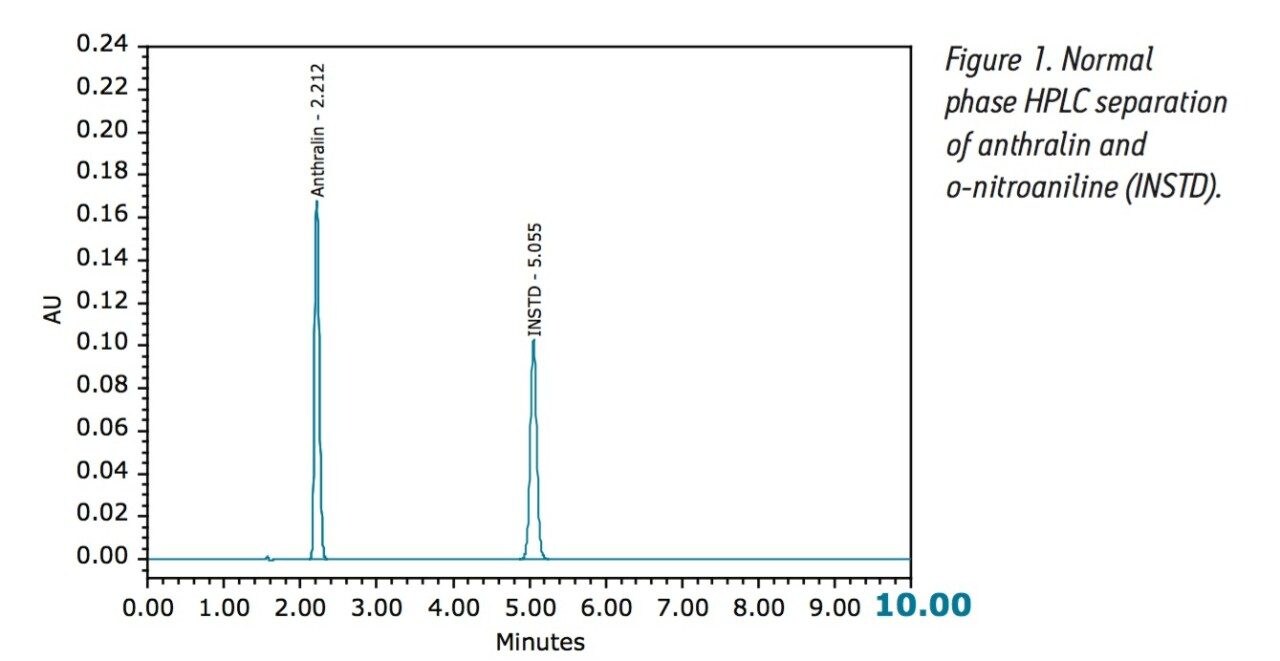
Currently, the United States Pharmacopeia (USP) assay for the drug substance anthralin, (9(10H)-Anthracenone, 1,8-dihydroxy-1,8- Dihydroxy-9-anthrone [CAS #1143-38-0] is a normal phase HPLC method. The isocratic separation is done using a 4.6 x 250 mm silica column (L3) with a mobile phase that consists of a mixture of 82:12:6 n-hexane, dichloromethane, and glacial acetic acid at a flow rate of 2 mL/minute as shown in Figure 1. The run time of this current compendial method is approximately 10 minutes (2X of the last major peak). Although this method is rugged and reliable, many laboratories have a desire to decrease the use of typical normal phase chromatography solvents (such as hexane and dichloromethane) for health, safety, environmental, and cost reasons.
Supercritical fluid chromatography (SFC) is a normal phase separation technique that uses carbon dioxide as the main mobile phase and often employs the use of polar modifiers such as methanol. Since the principles of SFC are similar to those of HPLC, methods should be able to be converted to SFC providing reduced solvent usage and disposal which will lower cost per analysis while enhancing health, safety, and environmental initiatives.
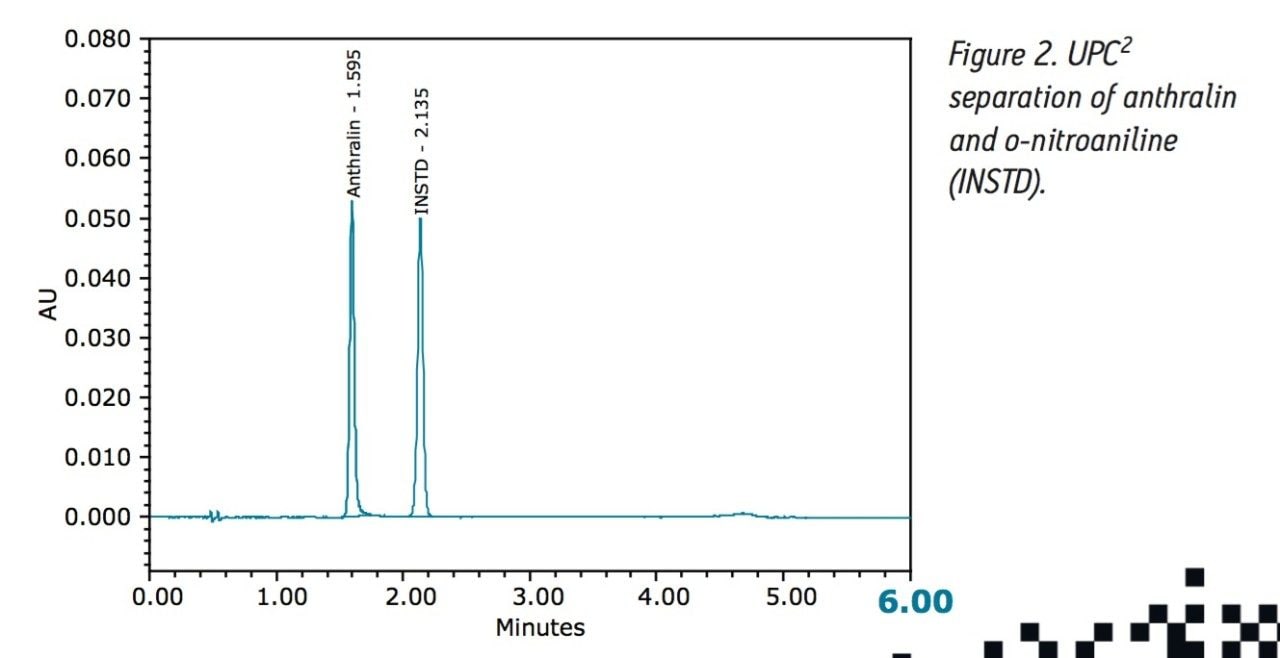
Methods converted to an SFC solution must maintain data quality (retention time reproducibility, resolution between compounds of interest and other components in the sample) and must produce results that are equivalent to the current normal phase method.
A sample of anthralin was prepared and analyzed using the current USP method (this sample was also used for the UPC2 analysis). The results of this analysis were used to compare the results obtained with the method developed on an ACQUITY UPC2 System. The UltraPerformance Convergence Chromatography (UPC2) method conditions were as follows:
|
Column: |
Viridis Silica 2-Ethylpyridine, 4.6 x 150 mm, 5 μm |
|
Temp.: |
40 °C |
|
Mobile phase: |
95% Carbon dioxide: 5% methanol containing 0.25% glacial acetic acid |
|
Flow rate: |
3.5 mL/min |
|
Back pressure: |
150 bar |
|
Detection: |
UV /PDA at 351 nm |
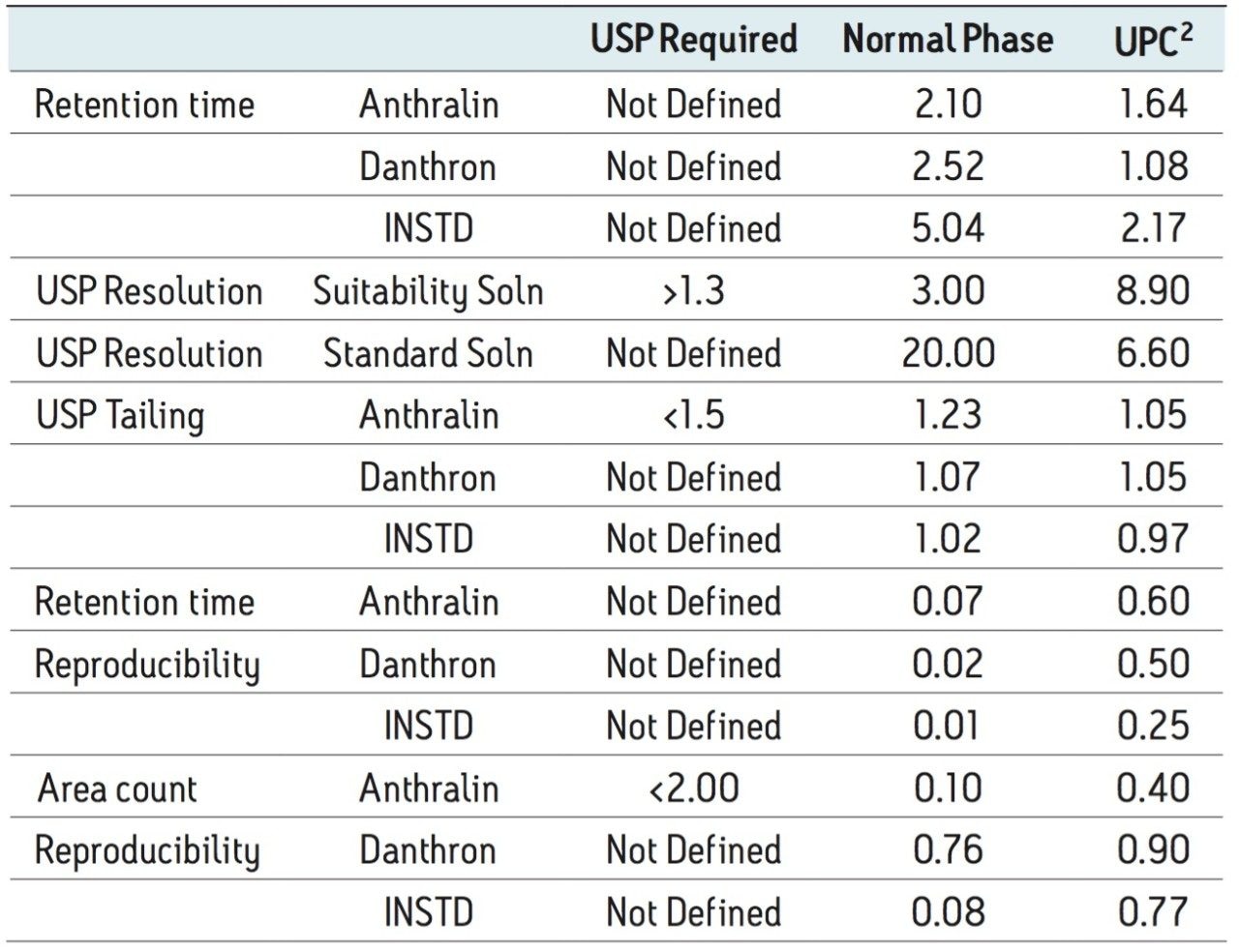
A comparison of key suitability parameters are shown in Table 1. In all cases, the results from the converted UPC2 method easily met or exceeded the required USP suitability values and were quite favorably comparable to the values from the normal phase method. Interestingly, there was a selectivity change between the two components of the suitability mix (anthralin and danthron) which did not negatively impact the results of the method conversion. The results of the analysis of an unknown purity anthralin sample were in good agreement between the two methods. The assayed anthralin sample contained 94.3% anthralin when analyzed using the normal phase HPLC method and 94.6% when analyzed by UPC2.
In this example, each normal phase HPLC run used 16.4 mL of hexane and 1.2 mL of dichloromethane. In contrast, the UPC2 method used only 1.05 mL of methanol. This demonstrates the significant reduction in organic solvent use that can be achieved by moving normal phase methods to UPC2. Based on current solvent prices, each normal phase HPLC run costs roughly $0.90 per run compared to $0.05 for each UPC2 run.
Using the ACQUITY UPC2 System, a USP compendial HPLC method was successfully converted to a UPC2 method. This new UPC2 method produced data of equal or better quality than the current HPLC method, was 1.6 times faster, and consumed less solvent. When high quality results are produced faster, laboratory productivity increases and cost per sample decreases. The ACQUITY UPC2 System is an ideal solution for laboratories converting their current normal phase HPLC methods to more efficient and cost effective UPC2 methods while enhancing health, safety, and environmental concerns.
720004236, February 2012