This application note discusses the effects of chromatographic system operating pressures on the performance of these sub-2 µm particle columns, and how a holistically-designed system maximizes their separation power.
The use of sub-2 μm particles in combination with the ACQUITY UPLC System provides superior performance for developing new separation methods or transferring existing HPLC methods to UPLC technology
High Performance Liquid Chromatography (HPLC) has become the workhorse of many analytical chemistry laboratories. HPLC is used in diverse applications, including pharmaceutical R&D, pharmaceutical product quality testing, clinical diagnostic testing, environmental analysis, and food and beverage safety analysis. This is mainly due to HPLC’s analytical versatility, ease of use, sample compatibility, and the selectivity and resolving power of the chromatographic process.
The chromatographic separating power of an HPLC system depends upon the selectivity of the mobile phase/stationary phase system and the efficiency of its column. Column efficiency is dependent upon two factors: column length and packing particle size (dp). Column efficiency increases with the square root of the column length and is inversely proportional to the particle size.
As chromatography has developed over the last 30 years, we have seen reductions in column particle size, from irregular 20 μm particles in the 1970s, to spherical 10 μm particles in the early 1980s, to 5 and 3 μm materials in the 1990s, to the variety of sub-2 μm column chemistries that have been commercially available since 2004.
Modern chromatographers today face challenging pressures on their laboratory: to increase productivity while also dealing with more and more complicated separation problems. They need to meet increasingly rigorous scientific demands while aligning with business drivers: from product innovations, to cost reductions, to faster sample processing times, to increased profitability. These novel sub-2 μm particle columns offer the most promise for LC to meet such disparate challenges without sacrificing performance.
The use of sub-2 μm particle columns has seen increased popularity since their introduction, however these materials produce higher backpressures than conventional 5 and 3 μm columns. This application note discusses the effects of chromatographic system operating pressures on the performance of these sub-2 μm particle columns, and how a holistically-designed system maximizes their separation power.
The chromatographic performance of columns with particle sizes ranging from 1.7 to 5 μm was investigated in both gradient and isocratic modes using a variety of test probes and conditions.
|
LC Systems: |
Waters Alliance HPLC 2695 System (HPLC) Waters ACQUITY UPLC System (UPLC) |
|
Column Temp.: |
30 °C |
|
Mobile Phase A: |
10 mM CH3COONH4 with 0.02% acetic acid |
|
Mobile Phase B: |
Acetonitrile |
|
Gradient: |
Hold at 2% B for 0.54 min, then to 15% B in 6.7 min, reset to 2% B at 7.94 min, equilibrate until 10.76 min (gradient for 4.6 x 150 mm, 5 μm) |
|
Injection volume: |
28.8 μL for HPLC; 2 μL for UPLC |
|
Detection: |
Photodiode Array (PDA) UV @ 254 nm |
|
Sampling rate: |
5 Hz for HPLC; 20 Hz for UPLC |
|
Time constant: |
0.1 |
Columns and flow rates are listed on their respective chromatograms. All flow rates and gradient conditions were scaled to dp.
The resolving power of different LC columns can be compared directly using the L/dp ratio of each (column length/particle size). For example, a 5 μm, 150 mm length column (L/dp = 30,000) has approximately the same resolving power as a 1.7 μm, 50 mm length column (L/dp = 29,412).
The data shown in Figure 1 illustrates the separation of a simple isocratic test mix using 5, 3.5, 2.5, and 1.7 μm columns. It is clear that the resolving power of each column is identical.
Also note that analysis time decreases as the particle size decreases. This is because shorter columns and higher linear velocities can be used with smaller particle columns without compromising chromatographic efficiency. The result is an analysis time that is 9X faster on the 1.7 μm column when compared to the 5 μm column.
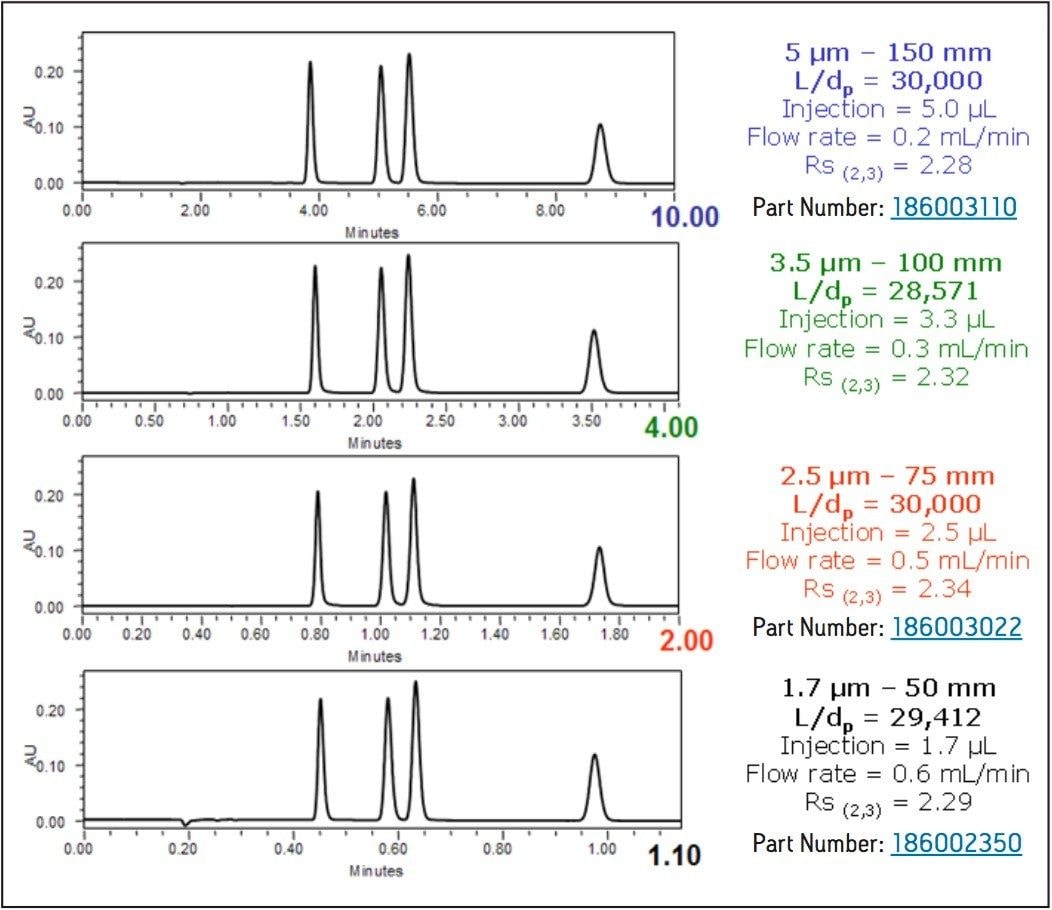
Figure 1. Isocratic separation of 1-methylxanthene, 1,3-dimethyluric acid, theobromine, and 1,7-dimethylxanthene (all 25 μg/mL) while maintaining a constant L/dp ratio. Peaks are listed in order of elution.
• 5, 3.5, and 2.5 μm columns: Waters XBridge C18.
• 1.7 μm column: ACQUITY UPLC BEH C18.
• All columns were 2.1 mm I.D.
• The isocratic mobile phase was 95% H2O, 5% ACN with 0.1% formic acid.
• The separation was performed at 38 °C; UV @ 280 nm.
Comparing the resolving power of different columns under gradient conditions is somewhat more complicated. Besides maintaining a constant L/dp ratio, it is also important to properly scale the gradient conditions based on column dimensions. For example, if the column length is decreased by a factor of two and the flow rate is increased by a factor of two, then the gradient duration should be decreased by a factor of four.
The data in Figure 2 show the separation of didanosine and its impurities on 5, 3.5, and 2.5 μm columns using conventional HPLC instrumentation. When the separation is scaled from the 5 μm column to the 3.5 μm column, the L/dp ratio is kept constant, and peak capacities are nearly the same.
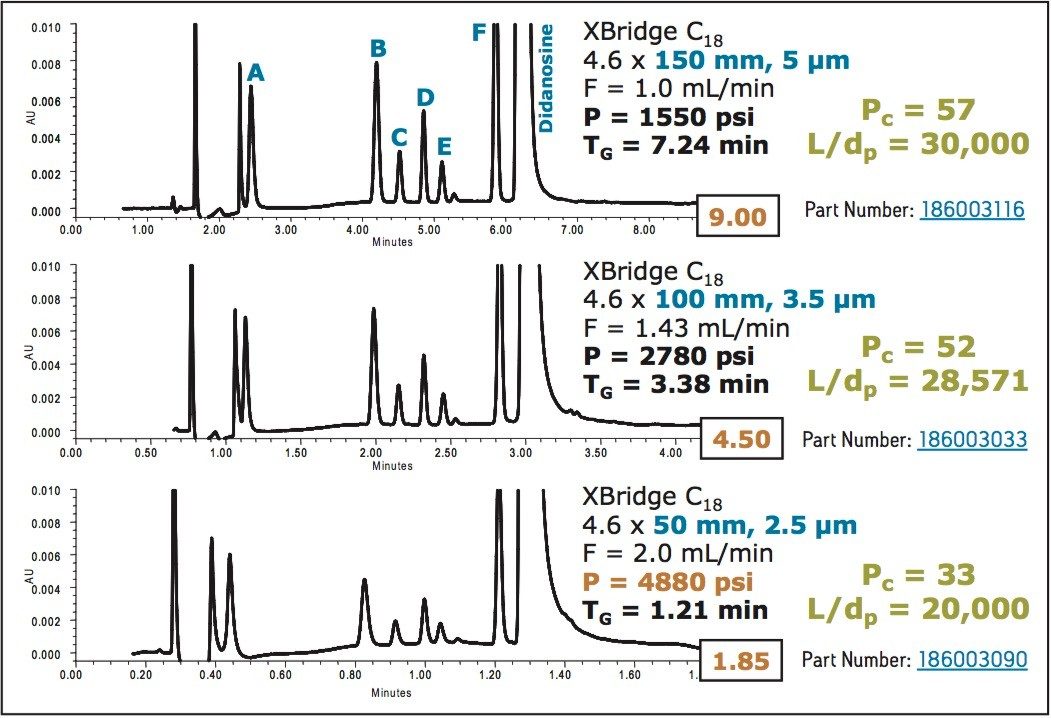
When the separation is transferred to the 2.5 μm column the peak capacity decreases significantly. This is because the conventional HPLC system is not capable of operating at the conditions needed to preserve the separation. A 75 mm length column is needed to maintain the same L/dp ratio; however, this length would cause the system to exceed the maximum system pressure (5000 psi). In order to use these smaller particle columns effectively and take advantage of their performance, a system with higher operating pressure is required.
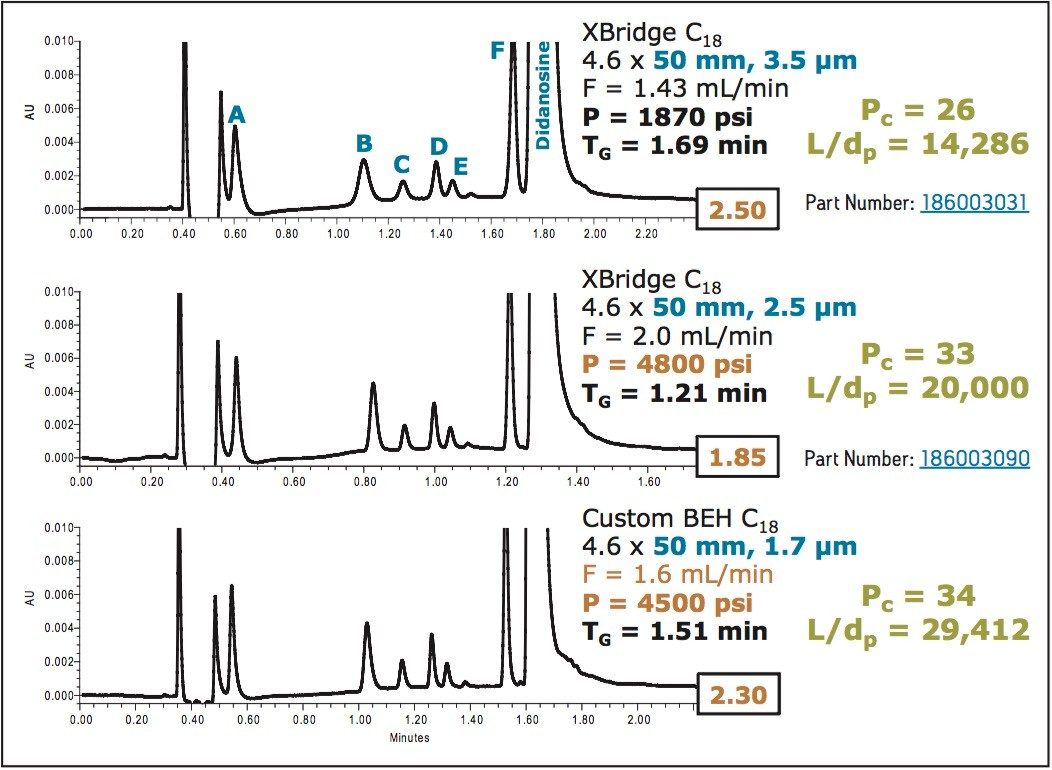
Besides reducing analysis time, smaller-particle LC columns can be used to increase overall resolving power in a chromatographic system. An incremental gain in performance should be seen by decreasing particle size and keeping column length constant.
Figure 3 shows that as the particle size is reduced from 3.5 to 2.5 μm, there is an 18% increase in peak capacity, which correlates well with the theoretical expectations. This is not the case with the 1.7 μm column. Pressure limitations of the conventional HPLC system do not allow its operation at the properly-scaled flow rate of 2.94 mL/min.
These data, combined with those in Figure 2, illustrate that in order to exploit the chromatographic potential of these sub-2 μm particle columns, it is necessary to run at pressures above the operating range of conventional HPLC systems.
Extended pressure capability is not the sole requirement when designing a chromatographic system to work with sub-2 μm particles. These materials are packed into narrow-bore columns (< 2.1 mm I.D.), and the chromatographic peaks produced are extremely narrow.
Therefore, the volume of the LC system itself must be carefully controlled in order to minimize peak dispersion and prevent loss of performance. This includes minimizing pre-column dispersion of the injected sample and post-column peak broadening prior to detection.
For gradient separations, a large system volume can result in a more significant gradient delay, which can decrease separation power and add unnecessary time to the analysis. Conventional HPLC systems have delay volumes in the range of 700 to 1450 μL. These systems can be altered and configured to reduce this volume somewhat by removing or bypassing some of the fluid paths (mixer, pulse damper, etc.). However, this can result in a loss of signal-to-noise for critical peaks present at low levels (Figure 4A). This is due to an increase in baseline noise caused by removal of critical components in the mobile phase pump.
The ACQUITY UPLC chromatography system has been purposely designed to operate with sub-2 μm particle columns. It has the capability of operating at pressures up to 15,000 psi (~1,000 bar) and has a system volume that is an order of magnitude lower than conventional HPLC systems. Thus no system modification is needed to realize the LC benefit of small column particles, and baseline noise is minimized.
The result is that the native UPLC system shows a 25X improvement in signal-to-noise over a modified HPLC system, which is especially important when detecting and quantifying low-level impurity peaks in a mixture (Figure 4B).
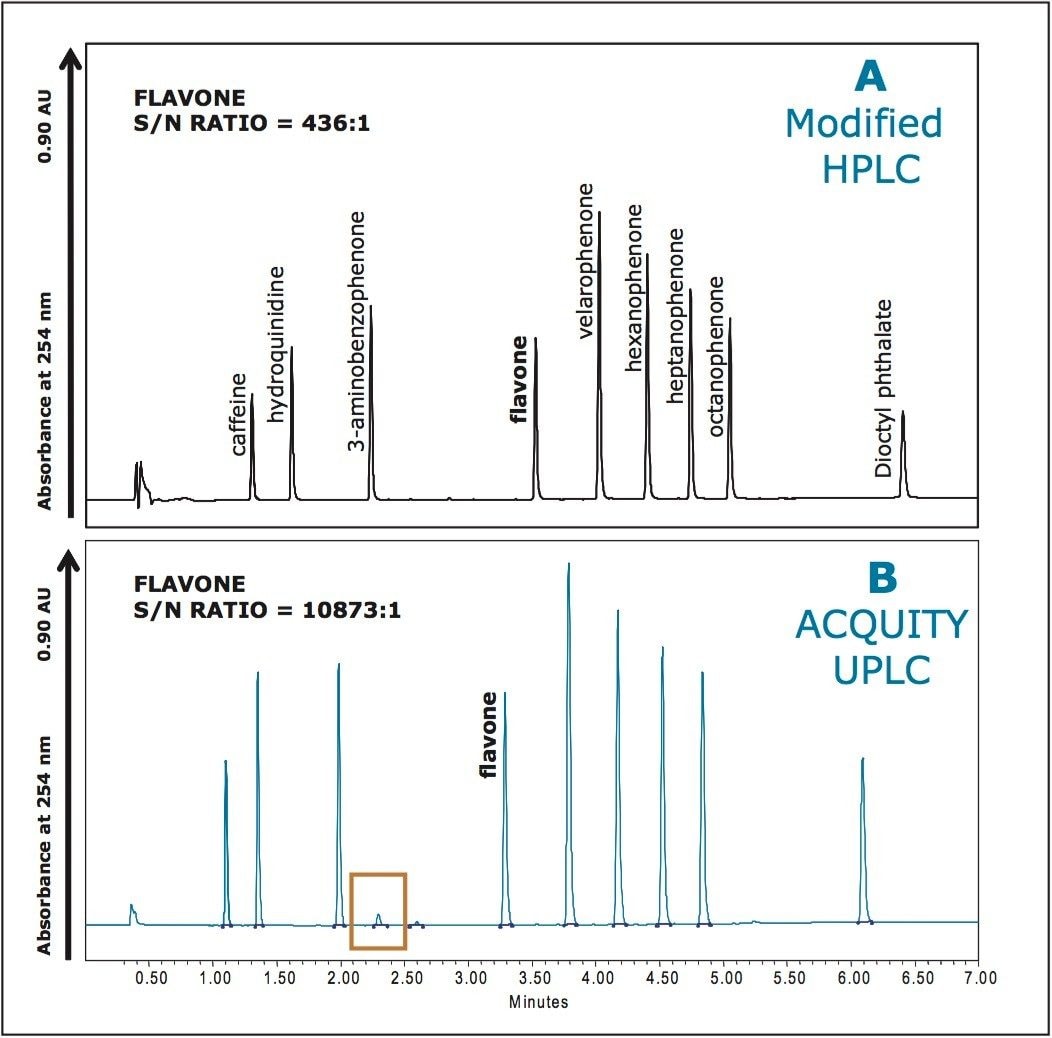
Figure 4. Comparison of baseline noise and instrument sensitivity using a modified HPLC system (A) versus the ACQUITY UPLC System (B).
• Mobile phase A was 0.05% TFA in H2O, mobile phase B was 0.05% TFA in ACN.
• Gradient from 5 to 95% in 5.17 min, hold for 1.7 min, reset.
• Flow rate 0.6 mL/min, 50 °C, UV @ 254 nm.
Sub-2 μm particle columns provide significant benefits in terms of productivity and chromatographic performance simply because shorter columns and faster flow rates can be used without adversely effecting performance. However, these columns can be used in such a way as to maximize the full potential of UPLC technology.
From the van Deemter equation we understand that the minimum plate height decreases as particle size decreases (Figure 5). In addition, the optimal linear velocity range increases as particle size decreases, which allows us to use sub-2 μm particle columns at even higher linear velocities without loss in chromatographic performance.
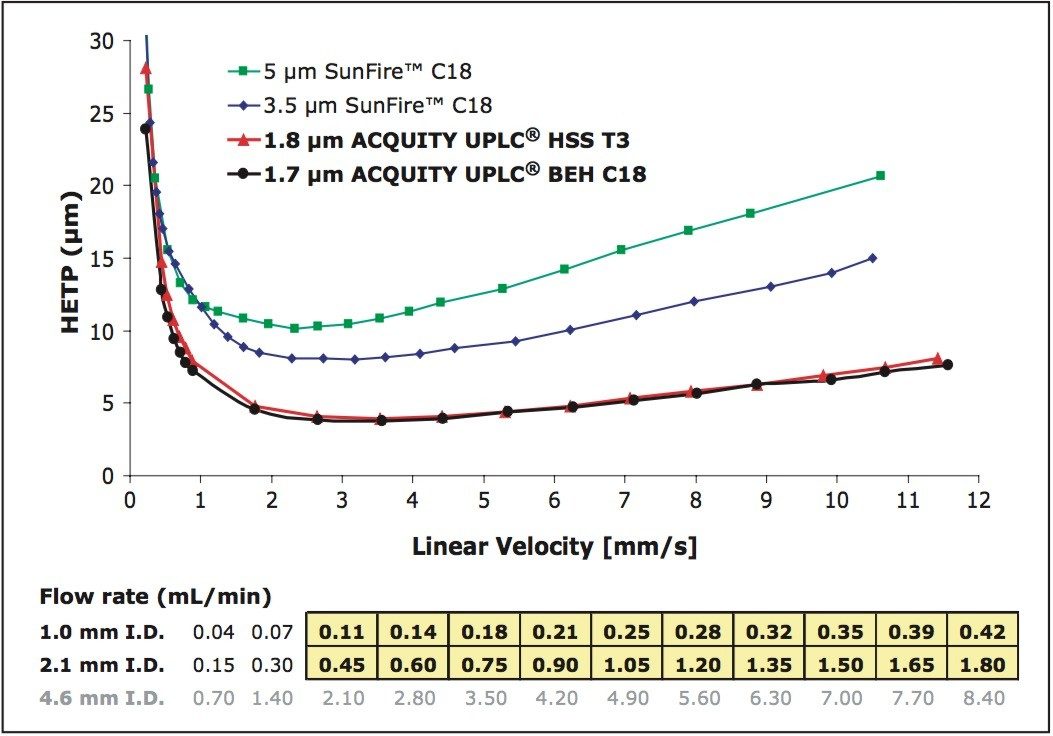
Figure 5. van Deemter plots for 5, 3.5, 1.8, and 1.7 μm particle columns.
• Test probe is heptanophenone.
• Mobile phase is 70:30 ACN/H2O.
• Temperature is 30 °C.
• All columns in the 2.1 x 50 mm format.
As mentioned previously, this necessitates an LC system that is capable of operating at higher backpressures. Once this limitation has been overcome, as it has been with the design of the ACQUITY UPLC System, small particle columns can be used at elevated flow rates to generate either ultra-fast or ultra-high resolution separations.
Ultra-fast separations can be performed by increasing the flow rate and proportionally decreasing the gradient time to keep a constant column volume in all steps of the gradient. Ultra-high resolution separations can be achieved by increasing flow rate and keeping analysis time constant.1
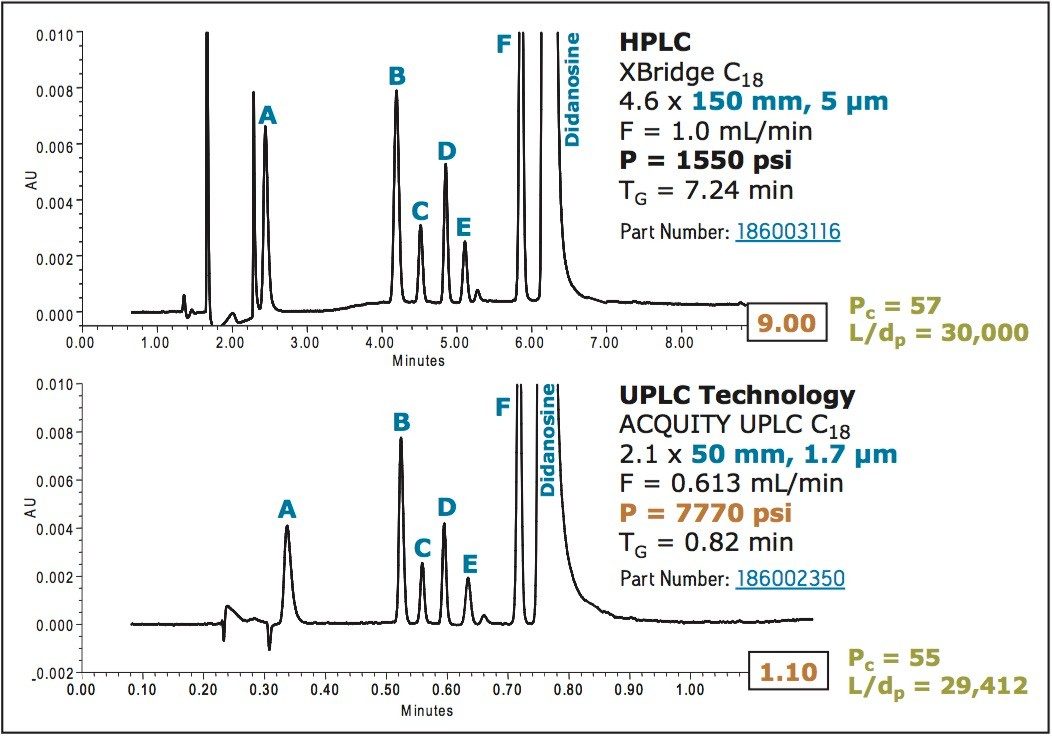
Examples of these two approaches can be seen in the following figures. Figure 6 shows the directly-scaled separation of didanosine and its impurities on a 5 μm and 1.7 μm column. In this example, the L/dp ratio remained constant, the flow rate was scaled according to dp, and the gradient times were adjusted based on column volume. Note that the peak capacities achieved are the same in both cases, only the analysis time on the 1.7 μm column is 49 seconds instead of more than 7 minutes on the 5 μm column.
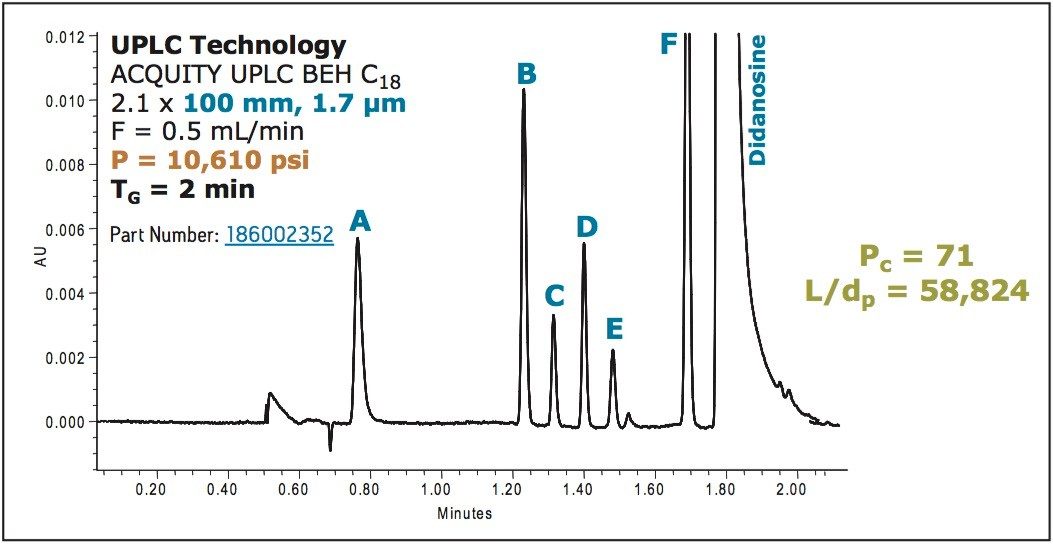
By increasing the column length to 100 mm (doubling L/dp), peak capacity was increased by 30% while maintaining a relatively short gradient time of 2 min (Figure 7), which is still more than 3.5X less than the original HPLC method shown in Figure 6.
Chromatographers today are challenged with producing more information, processing more samples, and returning higher-quality information in a shorter time frame. The development and commercialization of sub-2 μm porous particle LC columns has yielded the promised levels of performance with the added benefits of faster analysis time.
However, in order to take advantage of this increased performance, it is necessary to run sub-2 μm columns on a system specifically designed to maximize their full separation power.
This means that the system must be capable of operating at the backpressures generated by small particle columns operated at their optimal flow rate. It also means that the system volume must be minimized in order to prevent a loss in performance caused by band-spreading.
It is clear from the data presented here that a conventional HPLC system cannot be successfully modified to meet these criteria, and therefore cannot be used to realize the full potential of sub-2 μm particles.
The use of sub-2 μm particles in combination with the ACQUITY UPLC System provides superior performance for developing new separation methods or transferring existing HPLC methods to UPLC technology. The flexibility of this technology allows the chromatographer to optimize analyses for speed and productivity (with no loss in resolution), or for maximum resolution in the same analysis time.
720002619, April 2008