Plasmid Topology and Digest Analysis Using a GTxResolve™ 250 Å Slalom Chromatography Column
Jamuna Vaishnav, Balasubrahmanyam Addepalli, Matthew A. Lauber
Waters Corporation, United States
Published on August 18, 2025
Abstract
This application note illustrates the characterization of plasmid DNA topological isomers using a GTxResolve 250 Å Slalom Chromatography Column. Slalom chromatography discriminates large nucleic acid species by exploiting the dynamic differences in their rigidity and molecular stretching. Plasmid DNA provides the code for all recombinant expressions as well as a template for mRNA synthesis through in vitro transcription (IVT). Its quality is pivotal to ensuring the quality and efficacy of many different therapeutics, including mRNA drug substances. Purified plasmids can exist in supercoiled (S) as well as open-circular (OC) and linear (L) forms. Supercoiled species are desired for effective transfection when expressing recombinant proteins. Meanwhile, linearized plasmids are generally sought when performing IVT reactions to produce mRNA. In either case, impurities need to be kept to minimal levels. Here, the separation of plasmid isoforms in less than 5 minutes is shown by utilizing a slalom chromatography column manufactured from optimally packed high-purity, hydrophilic 2.5 µm particles and column hardware modified with MaxPeak™ High Performance Surfaces (HPS).
This column hardware and the packing material exhibit excellent inertness to produce high-resolution, high-recovery analyses. The identified chromatography conditions maximize the flow retardation effects of slalom chromatography to distinguish three different species of a large plasmid DNA of same size. Depending on the size of the plasmid, it is shown that slalom chromatography can potentially even separate closely related supercoiled and open circle plasmid species. Thus, slalom chromatography provides an intriguing new alternative to agarose gel electrophoresis or capillary gel electrophoresis to analyze plasmid DNA species in <5 minutes by resolving them. The ability of slalom chromatography to analyze plasmid DNA impurities should enable quicker development of efficacious and more globally accessible cell and gene therapy medicines.
Benefits
- Excellent separation of plasmid isoforms, including S and OC forms depending on the length of the constructs
- Confirmation of gene insert in recombinant plasmid using restriction enzyme digests
- Excellent analyte recovery with minimal nonspecific adsorption
- High-throughput analyses with gel-like separation capabilities for large (≥ 3kbp) nucleic acids
- Robust and tunable chromatography to maximize the separation conditions
Introduction
Genetic medicines use DNA and RNA to facilitate tailored medical interventions. Messenger RNA (mRNA) with protein-coding instructions can modify cellular gene expression through protein replacement or the introduction of novel proteins all together. Despite the promise and performance of mRNA drugs, the analytical strategies to maintain quality control are not fully developed or require tedious procedures.1 The large size, complexity, heterogeneity, and high molecular weight of most mRNA sequences, and lack of robust and high-throughput analytical methods, are slowing down the deployment of mRNA therapeutics. Key steps in the mRNA vaccine production include (i) preparation of a plasmid DNA template of protein coding sequence for IVT, (ii) IVT reactions for mRNA synthesis and mRNA purification, (iii) encapsulation of mRNA into a lipid nanoparticle, and (iv) sterile filtration before use. The quality of the plasmid that provides the template for mRNA synthesis is pivotal for ensuring the integrity and efficacy of the resulting mRNA. The plasmid may exist in the S, OC, and L forms due to nicks (cuts) on any one or both strands. The presence of OC and incorrectly linearized forms of a plasmid affects mRNA integrity as the IVT reaction gets terminated at random nicks, leading to shorter and heterogeneous transcripts.1 Uniform and appropriate linearization of the IVT plasmid is essential for efficient RNA synthesis and generating correct 5’ and 3’ ends. Thus, the analysis and quality control of IVT plasmid isoforms has become a critical strategy for minimizing process-related impurities in mRNA drugs. Current techniques for investigating these impurities, such as agarose gel or capillary gel electrophoresis can be tedious or require large quantities of samples. As such, alternative methods are desired.
Slalom chromatography is a promising alternative because it provides separation selectivity for large nucleic acids using pressure versus electrophoretic-driven effects. This chromatographic approach provides high-throughput analyses as a result of its inherently fast flow rates.3–4 In a slalom separation, the largest nucleic acid components and their topological isomers will experience flow retardation resulting in their separation. Large nucleic acids will undergo entropic extension when exposed to the shear forces exerted during a run. Due to their rigidity, linear double-stranded DNA (dsDNA) will resist these forces and attempt to revert to its equilibrium configurations. Any single-stranded species that might be present will succumb to these shear forces and rapidly pass through the column. Differences in the conformational states of supercoiled and linear plasmid DNA are also significant enough for them to be separated by slalom chromatography. Interestingly, mobile phase shear forces can be optimized to achieve the desired levels of selectivity in a separation. Flow rate, solvent viscosity, and column temperature all contribute to the optimization of slalom chromatography separation.
In this application note, the reproducible high-resolution separations of plasmid DNA isoform species is shown using GTxResolve 250 Å Slalom Chromatography Column. Large plasmid DNA isoforms do not access the intraparticle pore volume of this packing material with a 250 Å average pore diameter, but they are resolved across time and elution volume due to flow retardation differences. Optimization of column chromatography conditions, including column temperature, enabled the resolution of all three isoforms of an example plasmid DNA (≥ 7kbp) in under 5 minutes, making it a very high-throughput analysis method.
Experimental
|
1X TAE buffer: |
40 mM Tris-acetate, 1 mM EDTA pH 8.3 made from 10X TAE (ThermoFisher Technologies p/n: 15558042) |
|
Column storage: |
10% acetonitrile, 90% aqueous 25 mM Sodium Phosphate pH 7.0 + 100 mM Potassium Chloride |
|
Seal and weak wash: 20% Methanol: |
80% Water |
|
Samples: Plasmids: |
pBR322 (New England Biolabs – N3033L), pCMV-Cas9-2A-GFP (PP (CAS9GFPP-1EA) and pCMV-Cas9 (CAS9P-1EA) from Millipore Sigma. EcoRI (R0101S), NheI-HF (R3131S), and XbaI (R0145S) were procured from NEB. |
|
Plasmid digests: EcoRI digest of pBR322: |
To 43 µL of 18.2 MΩ*cm water, 5 µL of 10X NEBuffer (r2.1), 1 µL of pBR322 (NEB p/n: N3033L) and 1 µL of EcoRI (NEB p/n: R0101T) were added, vortexed, and briefly centrifuged to gather all the contents. After incubation at 37 °C for 1 hour, the enzyme was inactivated by incubating the digest at 70 ºC for 20 minutes. |
|
Double digest of pCMV-Cas9: |
To 34 µL of 18.2 MΩ*cm water, add 10 µL of pCMV- Cas9 plasmid (20 ng/µL) yielding a total of 200 ng of plasmid then add 5 µL of 10X rCutSmart™ Buffer, 0.5 µL of XbaI (NEB p/n: R0145S) and 0.5 µL of NheI (NEB p/n: R3131S) were added, vortexed and briefly centrifuged to gather all the contents. After incubation at 37 °C for 1 hour, the enzyme was inactivated by exposing the digest to 75 ºC for 20 minutes to inactivate the enzymes. |
|
Agarose gel electrophoresis: |
0.6% agarose gel was prepared by using Agarose (A0539-500G) from Sigma Aldrich, LOT# 0000320705, Source SLCR2997. About 0.6 g of agarose was dissolved in 100 mL of 1X TAE buffer by boiling in a microwave, after initial cooling (~1-2 minutes), 10 µL of SYBR® Gold Nucleic Acid gel stain (Invitrogen p/n:S11494) was added, thoroughly mixed and poured into a gel tray with a comb. After solidification at room temperature, 1X TAE buffer was added, the comb was gently pulled to make wells for sample loading. Load 200 ng of sample into individual wells. The gel setup in a horizontal Bio-Rad electrophoresis system was connected to the PowerPac HV High-Voltage Power Supply. Electrophoresis was done at 50V for 2 hours and 10 minutes. After electrophoresis, the gel was imaged using the Gel Doc EZ Gel Documentation System (Bio-Rad) to visualize the bands. |
|
LC system: |
ACQUITY™ UPLC™ I-Class Bio (or equivalent) consisting of QSM H-Class (Quaternary Solvent Manager with 50 µL mixer), TUV Detector - 1000 psi MAX Pressure 70 bars Max Pressure 500 nL, Sample Manager FTN (Ver. 2023 03 02), 15 µL Needle size (p/n: 700012820) Assy, Needle, FTN-15 µL, HPS, TXT w/Gd&st), 100 µL Sample syringe size, Injection Valve Flow Through (p/n: 700011791) External Pre-Heater, CH-30A, with Active Preheater (APH) MP35N, 0.004” ID, 18.5’’ (p/n: 430005558) and post column tubing to TUV: 0.004’’ ID 22.5’’ LG MP35N Welded Tube (p/n: 430002856), Assy, Pre-HRT to Column, Reusable MP35N (p/n: 700011809), ACQUITY UPLC 30 cm Column Heater/Cooler ( p/n: 176015127) |
|
Column: |
Waters™ GTxResolve 250 Å Slalom Column, MaxPeak Premier Technology, 2.5 µm, 4.6 x 300 mm and dsDNA 23K Ladder (p/n: 176006046) |
|
Mobile phase A: |
1X TAE buffer, pH 8.3 |
|
Column equilibration: |
Column conditioning is done by ramping the flow rate by 0.1 mL increments to reach the flow rate of 1.3 mL/min over 20 minutes and continued for another 40 minutes. |
|
Vials: |
Max Recovery Sample Vials (Waters p/n:186009186 (vial) 186009186 (caps)) |
|
Column temperature: |
5 °C, 25 °C, and 40 °C |
|
Sample temperature: |
5 ± 3 °C |
|
Sample amount: |
1 µL |
|
Flow rate: |
0.5 or 0.7 mL/min |
|
Gradient: |
Isocratic |
|
Sampling rate: |
40 Hz |
Results and Discussion
The capability of a GTxResolve 250 Å Slalom Column to produce fast yet reproducible separations of large DNA molecules was demonstrated in a previous application note.8 In the current studies, applying slalom chromatography to the characterization of plasmid samples was taken into consideration. Plasmid DNA, when purified from cells, exists mostly as a S configuration with varying amounts of OC and L forms as impurities. When transfecting cells, it is desired to have high-purity supercoiled DNA. When performing in vitro transcription reactions to produce mRNA, it is important to work with high-purity linearized DNA. It is critical that supercoiled DNA be cleaved at the designated restriction site for linearization to ensure appropriate 5’ and 3’ termini are obtained for synthesized mRNA drug substance.
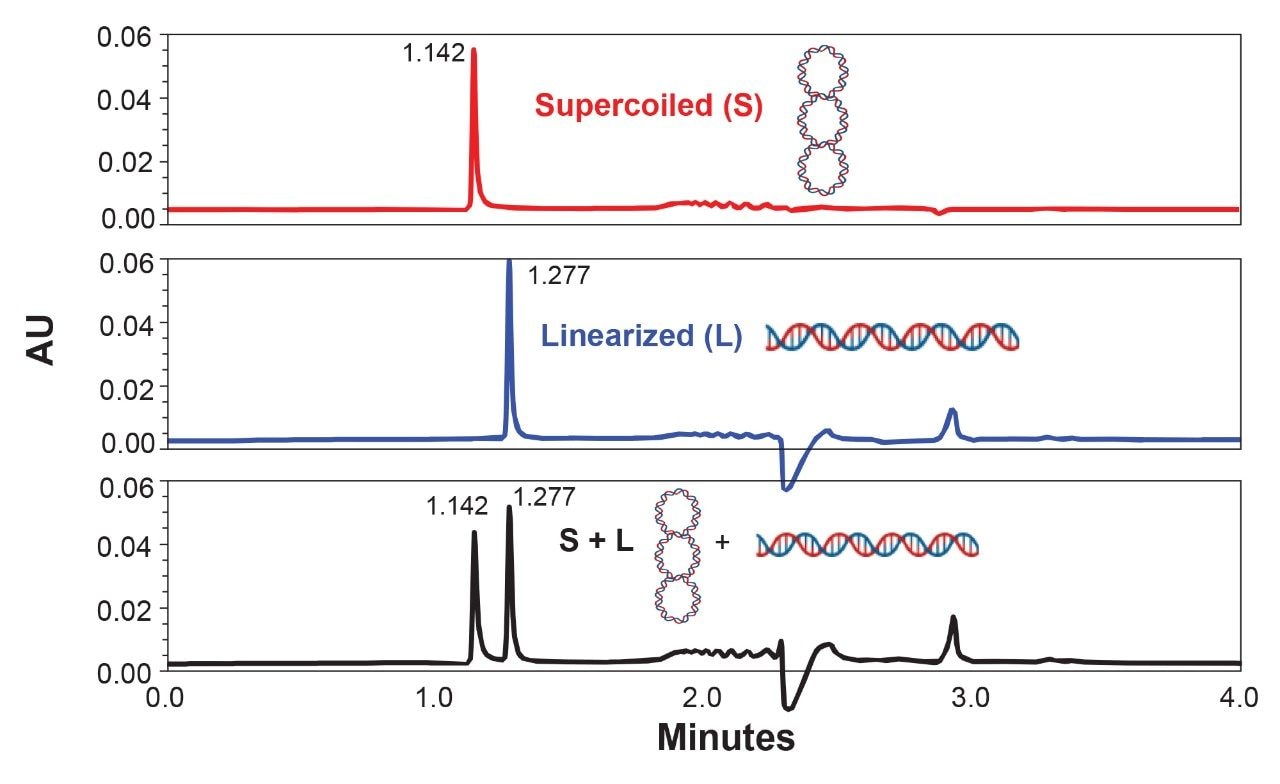
Figure 1 shows the ability of slalom chromatography to evaluate the extent of linearization of plasmid DNA in <5 minutes. In this example, E. coli plasmid pBR322 (4,361 bp) was examined. It harbors a single cleavage site for EcoRI at 4,359 bp; therefore, upon cleavage the supercoiled form will be converted into a linear form. Due to its rigid supercoiled structure, the untreated pBR322 is expected to differ in its stretching ability compared to the linearized version, thus differing in their flow retardation effects. As predicted, the supercoiled plasmid eluted early at approximately 1.142 minutes, while the linearized form was retained longer, leading to their elution at ~1.277 minutes. This pattern was reproduced again when a mixture of supercoiled and linearized forms of pBR322 was subjected to slalom chromatography (Fig 1; bottom panel). Thus, if plasmid preparations involve linearization, analysts can easily apply slalom chromatography to measure percent linearization.
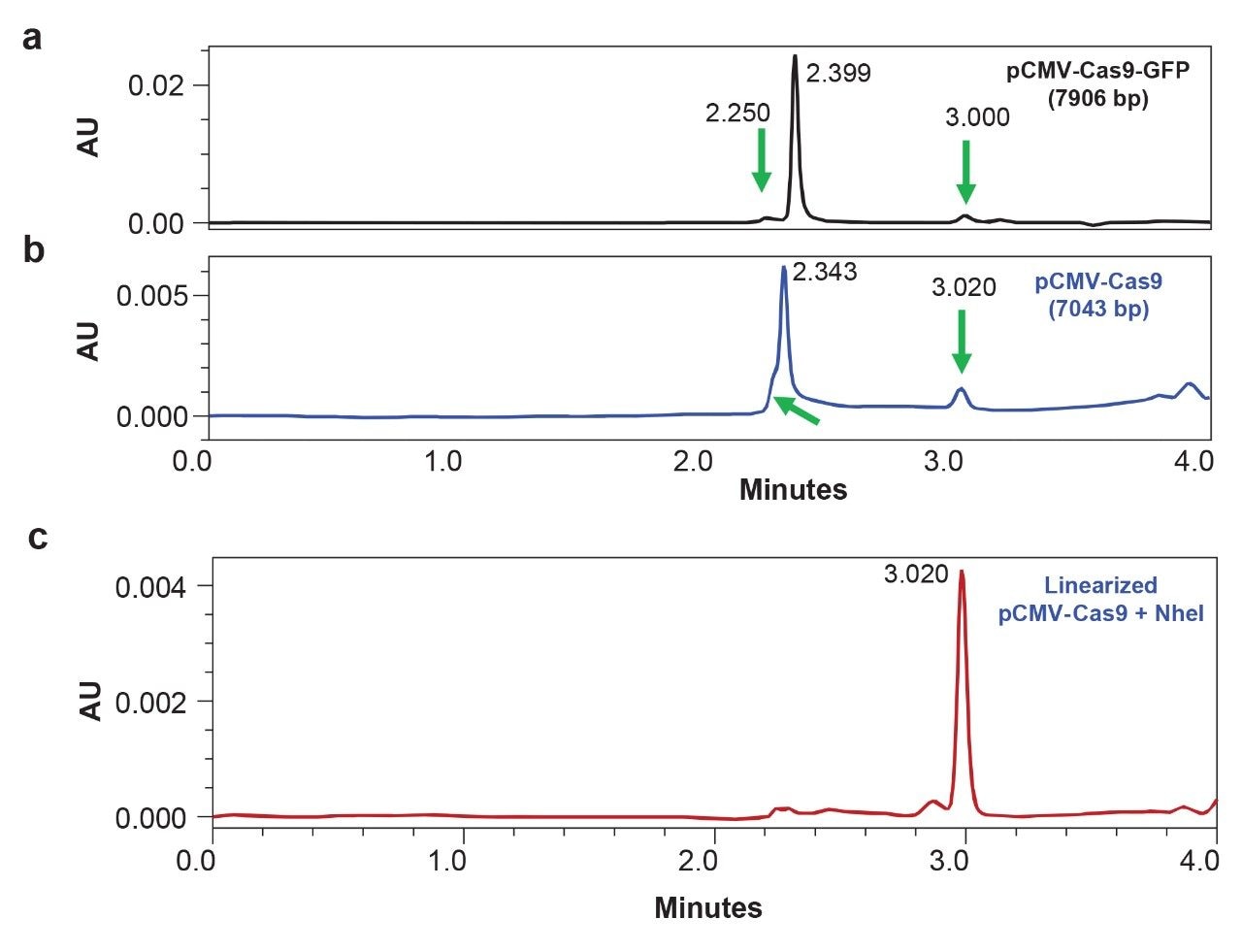
To investigate whether an open circular form of a plasmid can be detected by slalom chromatography, the refrigerated column temperatures and a set of plasmids of varying sizes was examined. Separations were performed at 5 °C column temperatures using an auxiliary column heater/cooler oven. Although pBR322 did not show any significant effects at lower temperature (data not shown), larger size plasmids pCMV-Cas9-GFP (7906 bp) and pCMV-Cas9 (7,043 bp) exhibited multiple features, including pre-peaks before the main peak as well as a late eluting peak (Figure 2). Interestingly, the main peaks (RTs of 2.399 and 2.343 minutes for Cas9-GFP and Cas9, respectively) in both plasmids exhibit a retention time difference of 0.056 minute, reflecting the difference in their size. Further, NheI-treated pCMV-Cas9 exhibited a peak that corresponded to the late eluting peak of the undigested plasmid. The linearized sample of pCMV-Cas9 (Figure 2c) also exhibited additional features, namely small peaks at a retention time window corresponding to supercoiled plasmids. These observations provide further evidence that the extent of plasmid digestion and generation of any potential artifacts can be monitored by slalom chromatography as long as the plasmid sample is greater than or equal to 7 kbp in size. Smaller (<7 kbp) plasmid samples will require the use of anion exchange chromatography to effectively resolve supercoiled and open circular variants. Simultaneous use of anion exchange and slalom shearing forces is also promising, as recently reported by Finny et al.9
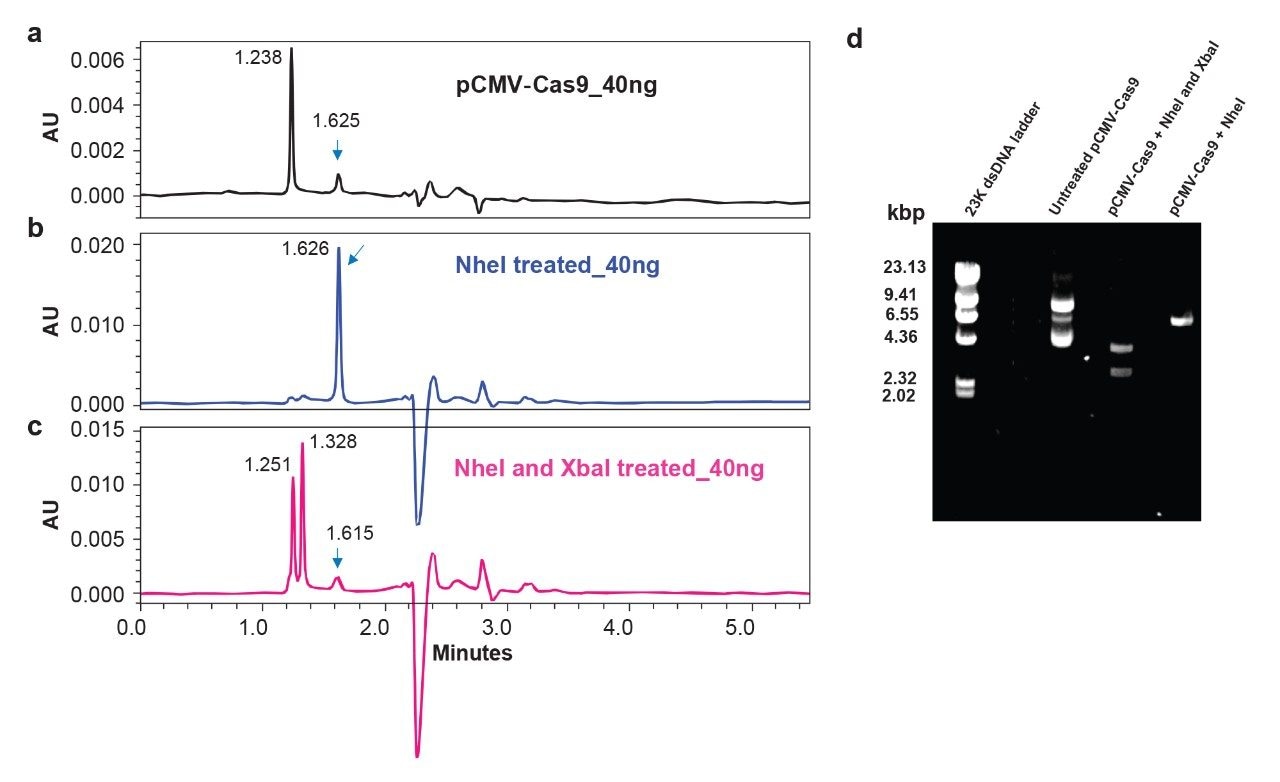
Slalom chromatography can also be applied for another type of investigation: confirming the insertion of a gene of interest. Successful cloning of a gene sequence into the designated sites of a plasmid backbone is generally monitored by agarose gel electrophoresis followed by double digestion with two restriction endonucleases. Figure 3 shows that slalom chromatography can be used to monitor the presence of a gene insert in less than 5 minutes. Double digestion of pCMV-Cas9 was performed by using NheI and XbaI (panel c) and compared against undigested (panel a) as well as linearized (NheI treated) plasmid (panel b). Successful cleavage of pCMV-Cas9 at NheI and XbaI sites was confirmed to release the 4147 bp Cas9 gene sequence. Although the linear Cas9 insert (4147 bp) and the residual plasmid backbone (~2896 bp) exhibit a relatively small difference in length (1259 bp), they can still be resolved (a difference of 0.077 min) by slalom chromatography indicating its sensitive detection capability to evaluate digestions as compared to agarose gel electrophoresis (Figure 3d). About 200 ng of digest were needed to see clear bands for gene insert and plasmid backbone on an agarose gel. In contrast, 40 ng of digest were sufficient to detect both the peaks, indicating 5X improvement in sensitive detection by slalom chromatography.
Conclusion
Slalom chromatography by GTxResolve 250 Å Slalom Column is highly suitable for plasmid analysis at various steps in both cell culture processing and cell-free syntheses.
a. Extent of plasmid linearization before synthesis of mRNA drug substance by in vitro transcription.
b. Plasmid quality evaluation to detect the levels of open circular and linear forms of impurities on constructs ≥7 kb.
c. Confirmation of gene inserts in recombinant plasmids by digestion with appropriate restriction endonucleases.
d. Sensitive detection of plasmid digests with 5x improvement compared to agarose gel electrophoresis.
As such, slalom chromatography with a GTxResolve 250 Å Slalom Column provides a viable high-throughput alternative to tedious agarose gel electrophoresis methods. Its quick readout on plasmid DNA samples in <5 minutes will be helpful to a number of different molecular biology and process development laboratories.
References
- Du, J. et al. The rise of mRNA therapeutic vaccines. RSC Pharm. (2025) 2, 235–256. https://doi.org/10.1039/d4pm00309h.
- Piao, X et al. Supercoiled DNA percentage: A key in-process control of linear DNA template for mRNA drug substance and manufacturing. Molecular Therapy Nucleic Acids (2024) 35, 102223. https://www.sciencedirect.com/science/article/pii/S2162253124001100?viewFullText=true
- Boyes, B., Walker, D., McGeer, P. Separation of large DNA restriction fragments on a size exclusion column by a non-ideal mechanism, Anal Biochem 170, 127–134 (1988). https://doi.org/10.1016/0003-2697(88)90099-1.
- Hirabayashi, J., Kasai, K. Slalom chromatography: Size-Dependent Separation of DNA Molecules by a Hydrodynamic Phenomenon. Biochemistry 29, 9515–21 (1990). https:// 10.1021/bi00493a004.
- Gritti, F., Wyndham, K. Retention Mechanism in Combined Hydrodynamic and Slalom Chromatography for Analyzing Large Nucleic Acid Biopolymers Relevant to Cell and Gene Therapies. J Chrom A 1730:465075 (2024) https://doi.org/10.1016/j.chroma.2024.465075.
- Gritti, F. Ultra-High Pressure Slalom Chromatography: Application to the Characterization of Large DNA and RNA Samples Relevant in Cell and Gene Therapy. J Chrom A 1738, 465487 (2024). https://10.1016/j.chroma.2024.465487.
- Gritti, F. Retention Mechanism in Slalom Chromatography: Perspectives on the Characterization of Large DNA and RNA Biopolymers in Cell and Gene Therapy. J Chrom A 1743, 465691 (2025) https://doi.org/10.1016/j.chroma.2025.465691.
- Vaishnav, J. et al. A New Alternative to Gel Electrophoresis: Higher Resolution and Faster Analysis of Large Nucleic Acids by Rigorously Designed GTxResolve™ 250 Å Slalom Columns. Waters Application Note: 720008921.
- Finny, A. et al. Slalom-Aided Anion Exchange Chromatography for Enhanced Analysis of Plasmid DNA Topological Impurities. Waters Application Note: 720008928.
720008954, July 2025