Comparing Column Lifetime of MaxPeak™ Premier Columns and Standard Hardware Columns
This is an Application Brief and does not contain a detailed Experimental section.
Abstract
This application brief examines the use of two columns, packed with the same stationary phase, for the analysis of formulated tablet samples. We performed 5,000 injections of the sample using traditional stainless-steel hardware and inert hardware, and critical peak and system attributes were monitored. After 5,000 injections, neither column showed any signs of fouling, as the system pressure and peak shape for the analyte were within 5% of initial values.
Benefits
- 5,000 injections of a formulated drug were carried out without any column clogging or performance loss
- Comparable lifetimes were observed for both the stainless-steel and inert hardware columns for the analysis of formulated tablet samples
Introduction
HPLC columns are critical components for a variety of workflows. Whether they are used in drug discovery or for quality control of formulated pharmaceuticals, having a column with a long lifetime is preferred, not only for financial reasons but also for scientific reasons. Having a column last longer means less variability caused by different sorbent lots, and while most validated methods go through extensive lot-to-lot robustness studies, having less variability in data over the lifetime of a product is always preferred.
The typical HPLC column on the market uses a stainless-steel column body and frit, However, some analytes have been shown to interact with stainless-steel surfaces, causing decreased peak area, peak broadening/tailing and high variability.1–4 The use of inert hardware for HPLC columns, such as MaxPeak Premier Columns, is still relatively new. MaxPeak Premier Columns have been shown to improve separations of several types of analytes, but until now, the lifetime of the columns when analyzing formulated samples had not been tested.1–4 This application brief examines a study where 5,000 injections of formulated naproxen tablets were processed and analyzed using a MaxPeak Premier Column as well as a stainless-steel column. System pressure and peak tailing were monitored during the injections, as those parameters were identified as the ones that typically fail first due to column fouling caused by particulate contamination on the column.5
Results and Discussion
Formulated naproxen tablets were prepared by crushing and diluting with a mobile phase consisting of acetonitrile:water:acetic acid (45:54:1) to a concentration of 1 mg/mL. The stock solution was then filtered through a 10 µm LC filter. After filtration, the stock solution was diluted to 0.1 mg/mL using mobile phase and placed on the system for analysis, using a 1 µL injection volume. To ensure column performance for both the stainless-steel and MaxPeak Premier Column, two critical attributes were monitored. The columns tested were 2.1 x 50 mm columns packed with CORTECS T3 2.7 µm sorbent. Both system pressure and peak tailing, as measured by USP tailing, were selected for monitoring as they are the most likely to be affected by column fouling due to particulates being caught on the column or in the stationary phase.
In order to assess the lifetime for both columns, 5,000 injections were performed using the formulated naproxen sample. Isocratic separations were performed, with a run time of 1.5 minutes per injection. This run time was sufficient to elute the analyte, while keeping the cycle time as low as possible to improve the throughput of the analysis. As mentioned previously, system pressure and USP tailing were monitored and plotted as percent change from initial for both columns. Figure 1 shows the plot of percent change in system pressure for both the MaxPeak Premier Column (blue) and the stainless-steel column (orange).
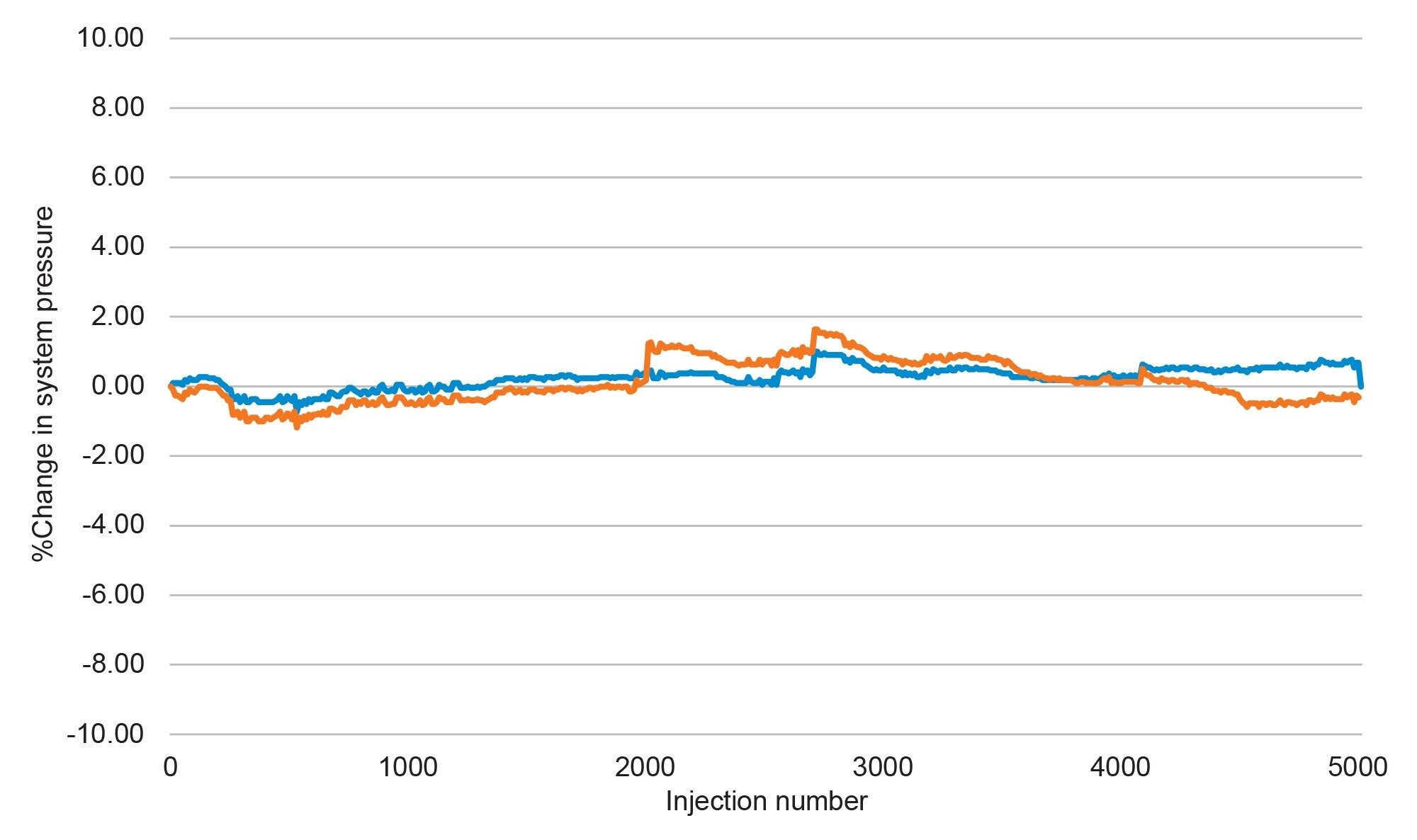
Over the course of the test both columns showed less than a 2% change in system pressure, which includes several mobile phase preparations which add potential variability in system pressure due to slight variations in organic percentage. Typically, when a column fouls due to particulate contamination from a sample, the system pressure will rise until either the system pressure exceeds the limitations of the system or the user stops the analysis and replaces the column. However, as shown above, there was no rise in system pressure indicating no particulate contamination. To further support this, peak tailing was also plotted as percent change from initial and is shown in Figure 2.
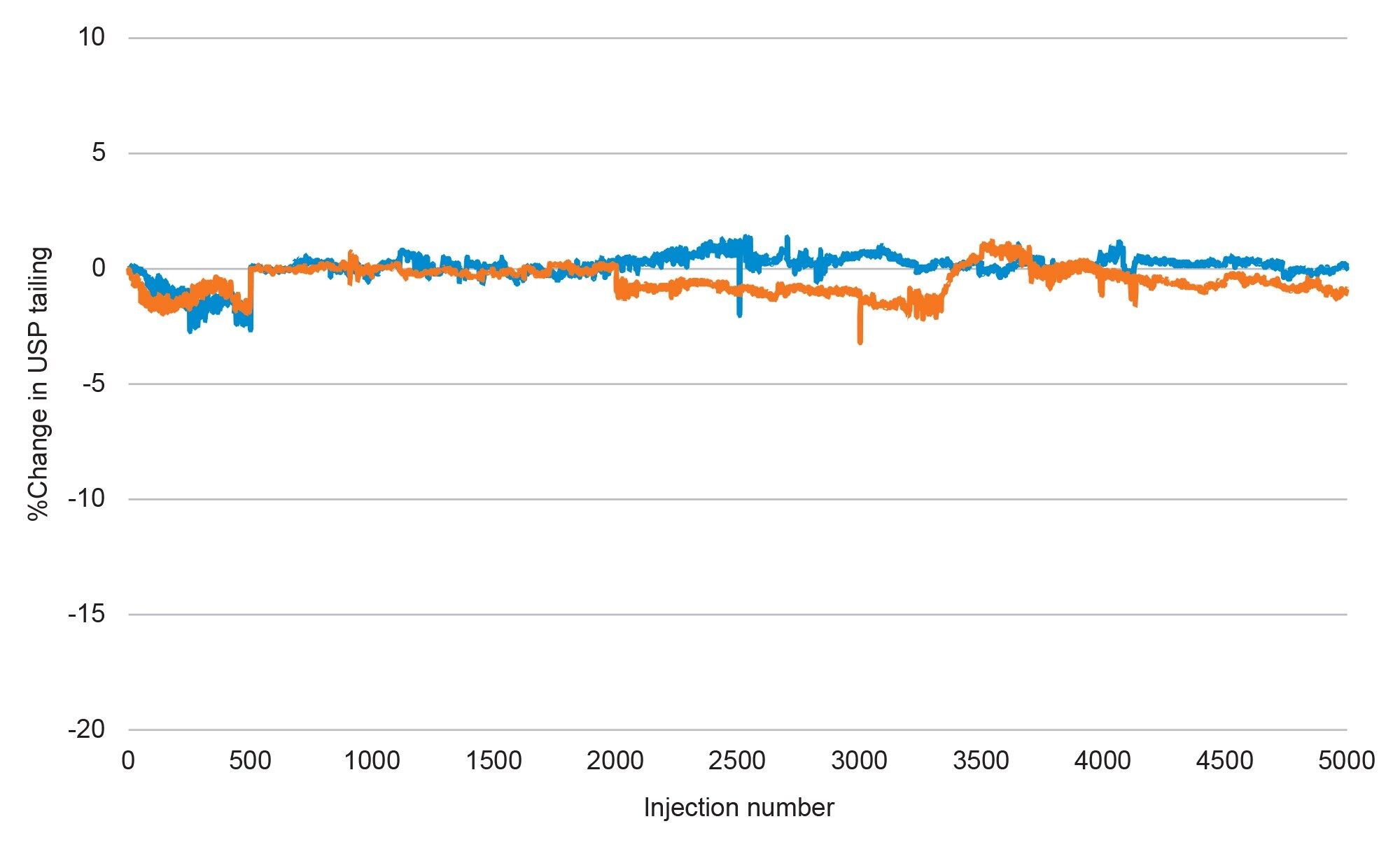
Similar to the results seen with system pressure, little change in USP tailing values for naproxen was observed over the course of the testing, with a less than 3% change in USP tailing over 5,000 injections. Peak shape is often impacted by particulates in the sample getting caught on the sorbent or in the inlet frit, disturbing how the analyte band moves through the column. As the data show no change in USP tailing, it can be reasonably assumed that the analyte bands are moving through the column in the same manner during injection 5,000 as they were during the first injection, indicating no significant accumulation of particulates on the frit or sorbent.
Conclusion
MaxPeak Premier Columns employ inert hardware to mitigate non-specific adsorption of analytes on the metal surfaces of the column. While this technology has benefits for some analytes, there may be a hesitation to adopt it as column lifetimes for formulated samples had yet to be examined. This application brief describes a 5,000-injection study performed on both a MaxPeak Premier Column and a stainless-steel column. Over the 5,000 injections, both columns showed no change in system pressure or peak tailing for the API, indicating that the MaxPeak Premier Column has a comparable lifetime to a stainless-steel column.
References
- Delano M, Walter TH, Lauber M, Gilar M, Jung MC, Nguyen JM, Boissel C, Patel A, Bates-Harrison A, Wyndham K. Using Hybrid Organic-Inorganic Surface Technology to Mitigate Analyte Interactions with Metal Surfaces in UHPLC. Anal. Chem. 93 (2021) 5773–5781
- Walter TH, Alden BA, Belanger J, Berthelette K, Boissel C, DeLano M, Kizekai L, Nguyen JM, Shiner S. Modifying the Metal Surfaces in HPLC Systems and Columns to Prevent Analyte Adsorption and Other Deleterious Effects. LCGC Supplements (2022) 28–34.
- Smith K, Rainville P. Utilization of MaxPeak High Performance Surfaces and the Atlantis Premier BEH C18 AX Column to Increase Sensitivity of LC-MS Analysis. Waters Application Note. 720006745, 2020.
- Jung MC, Lauber MA. Demonstrating Improved Sensitivity and Dynamic Range with MaxPeak High Performance Surfaces (HPS) Technology: A Case Study on the Detection of Nucleotides. Waters Application Note. 720007053, 2020.
- Berthelette K, Alden B, Osterman D, DeLoffi M, Turner J. Extending Column Lifetime using VanGuard Fully Integrated Technology (FIT) Column Protection. Waters Application note. 720007713, 2022.
Featured Products
720008690, February 2025