Soft drink analysis using the ACQUITY Arc System provides a simple method for the analysis of soft drink additives. Implementation of such a procedure in a manufacturing environment has the capacity to improve overall workplace efficiency. In this application note, we show that the Waters ACQUITY Arc System, XBridge Phenyl XP Columns, along with Waters Beverage Mobile Phase, can separate these compounds in under 10 minutes.
Soft Drink analysis using the ACQUITY Arc System, along with Waters Beverage Mobile Phase, XBridge Phenyl Column Chemistry, and a choice of Waters Beverage Standards, or Waters Big-4 Standard concentrate provides multiple benefits such as:
The soft drink market is an important revenue source for many major food and beverage producers. Such beverages include traditional carbonated soft drinks, high energy drinks, and recently popular healthy formulations such as vitamin waters and teas.
These products often contain caffeine as a stimulant, benzoate, and sorbate as preservatives; and for diet preparations, non-nutritive sweeteners such as acesulfame K, aspartame, and saccharin.
For quality control purposes the conformance of target concentrations of analytes to specified ranges is critical. In this application note, we show that the Waters ACQUITY Arc System, XBridge Phenyl XP Columns, along with Waters Beverage Mobile Phase, can separate these compounds in under 10 minutes.

|
LC system: |
ACQUITY Arc |
|
Runtime: |
10.0 min |
|
Column: |
XBridge Phenyl XP 2.5 μm, 4.6 x 50 mm |
|
Column temp.: |
35 °C |
|
Mobile phase: |
Waters Beverage Mobile Phase |
|
Flow rate: |
1.0 mL/min |
|
Flow path: |
1 (HPLC emulation) |
|
Injection volume: |
5 μL |
|
Detector: |
2489 UV/Visible (UV/Vis) |
|
Detection: |
214, 247 nm |
A: Beverage analysis standard (single point)
One bottle of Waters Beverage Analysis 5 Standards Solution, p/n 186006008, was poured into one bottle of Waters Beverage Analysis Standards Solid (aspartame), p/n 186006010, and mixed until all of the aspartame was dissolved. This resulted in a standard with a concentration of 150 mg/L acesulfame K, 100 mg/L saccharin, 200 mg/L benzoate, 100 mg/L sorbate, 100 mg/L caffeine, and 500 mg/L aspartame.
B: Standard concentrate (multi-point)
Waters Big-4 Calibration Stock Standard, p/n 186007980, (1000 mg/L each of acesulfame K, benzoate, sorbate, and caffeine) was diluted in water to produce eight separate levels with concentrations listed in Table 1.

Carbonated beverages were sonicated to remove carbon dioxide. All beverages were filtered through a 0.2-μm PVDF filter, then injected.
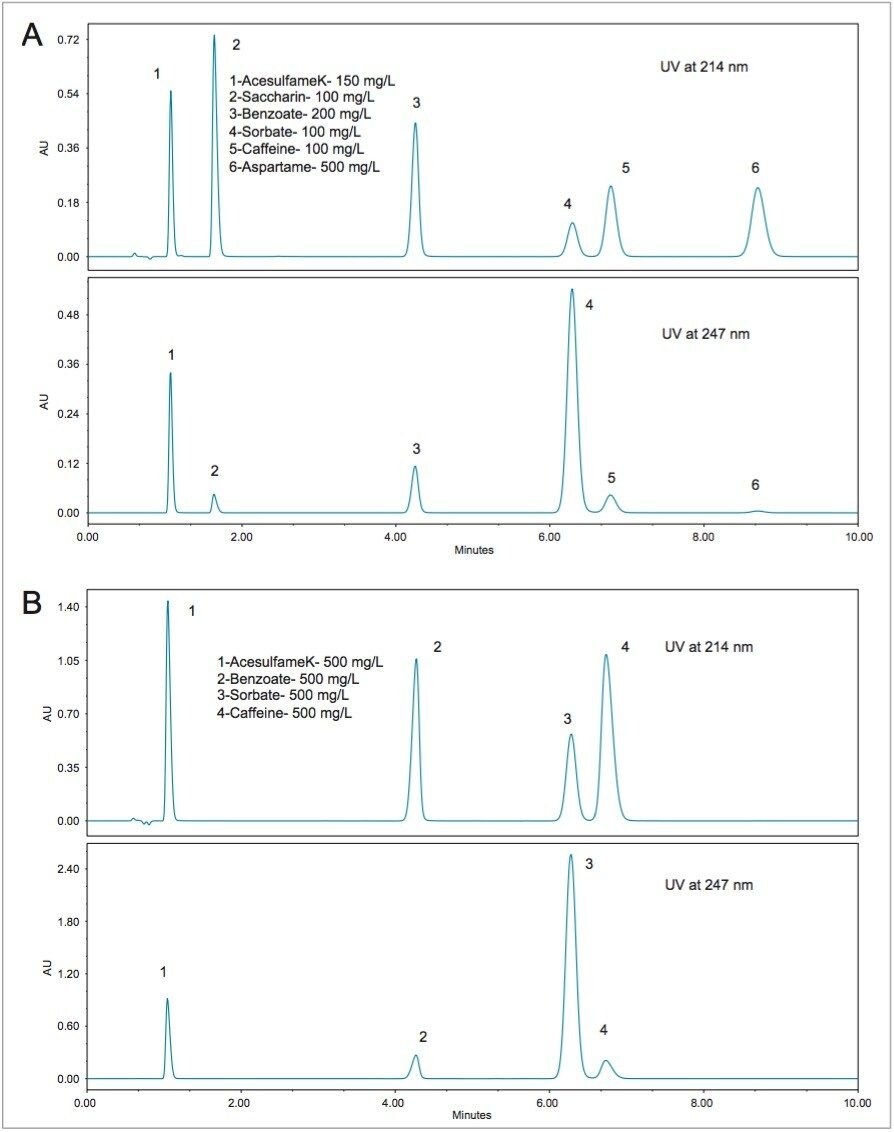
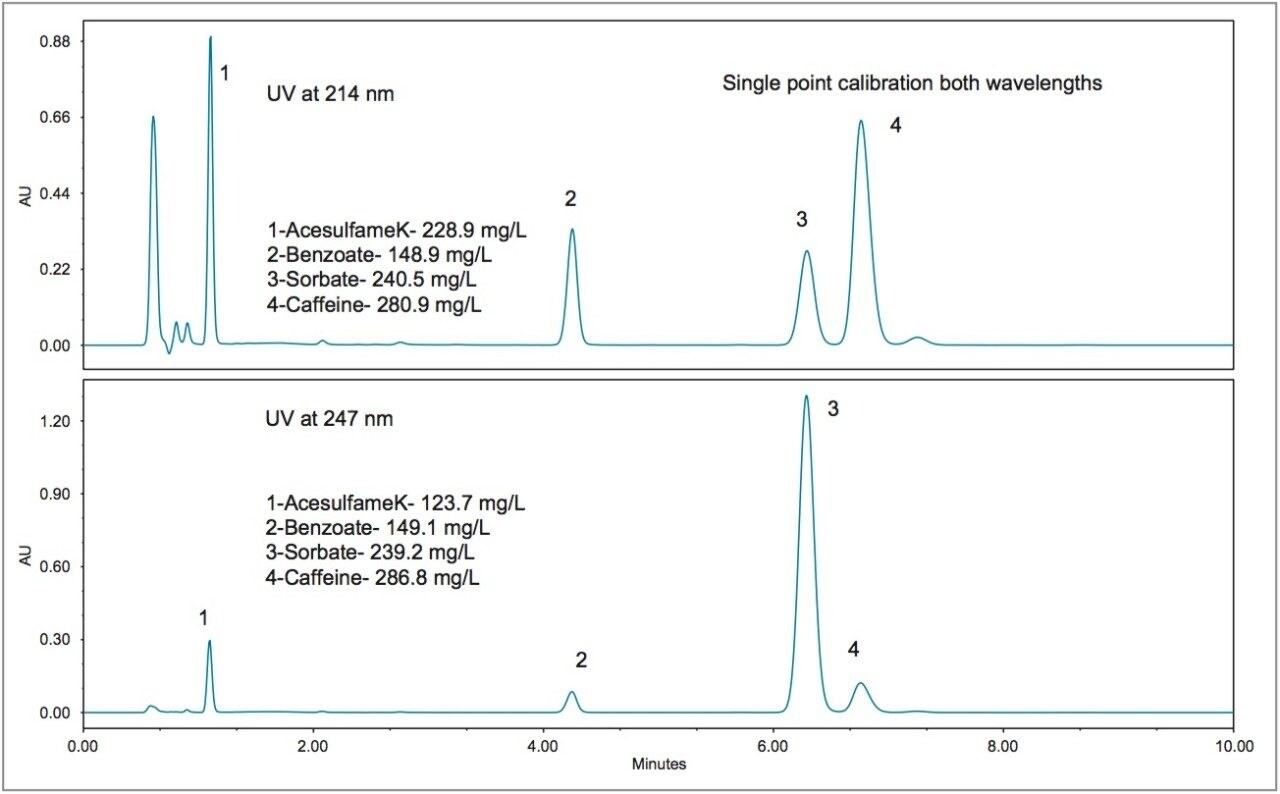
Chromatograms resulting from separation of beverage standards using the ACQUITY Arc System with an XBridge Phenyl XP Column are shown in Figure 2. Depending on the requirements of the analysis, there are two different options for the standards. The first standard, shown in Figure 2A, is the Waters Beverage Standard. This standard contains (in order of elution): acesulfame K, saccharin, benzoate, sorbate, caffeine, and aspartame. Each component has a different UV absorbance spectrum so that the peak height ratios are different as a function of the wavelength. As can be seen from the chromatogram in Figure 2A, two different wavelengths can be programmed and monitored in the same run. In Figure 2A and 2B chromatograms for both 214 nm and 247 nm are shown. Figure 2B shows the chromatograms for the Big-4 Standard, which has the same analytes as the Waters Beverage Standard, with the exception of saccharin and aspartame. These two sweeteners are not required for the analysis of beverages that do not use these ingredients.
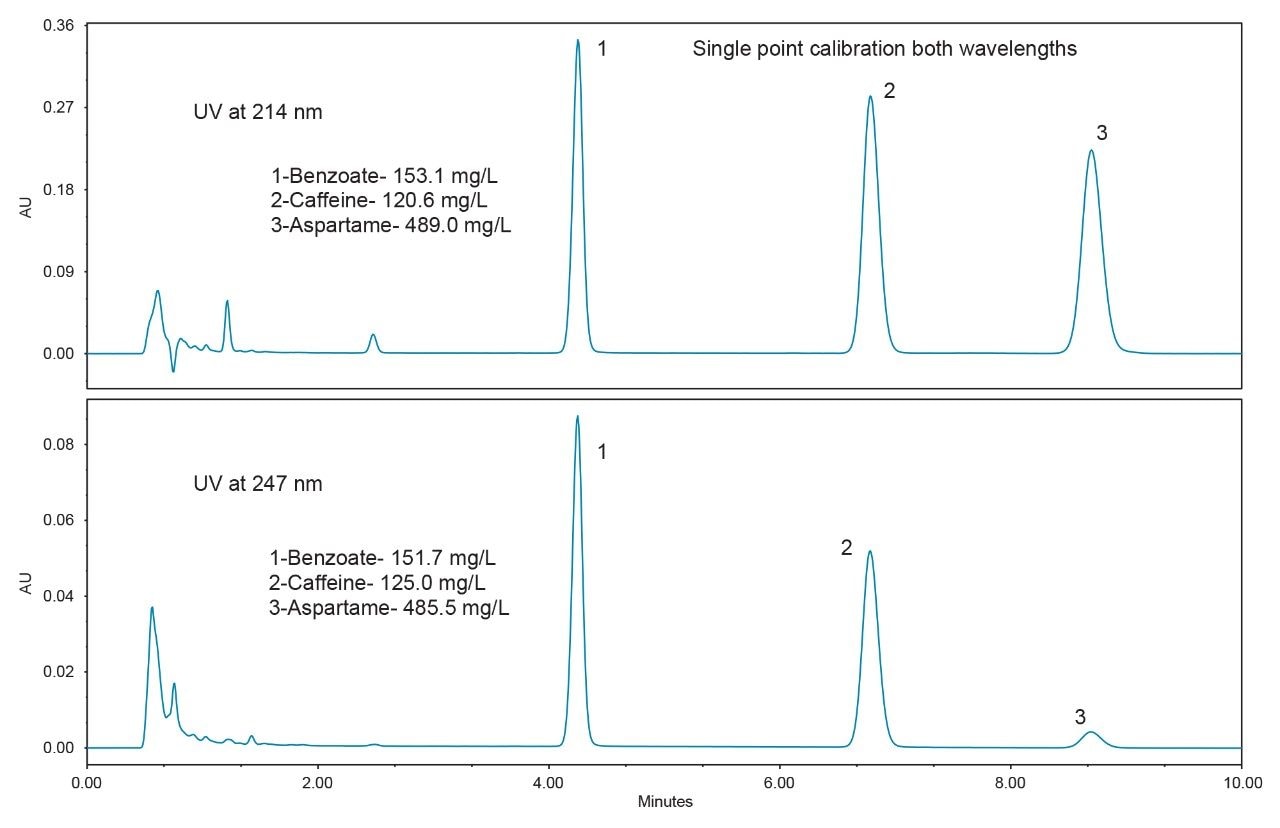
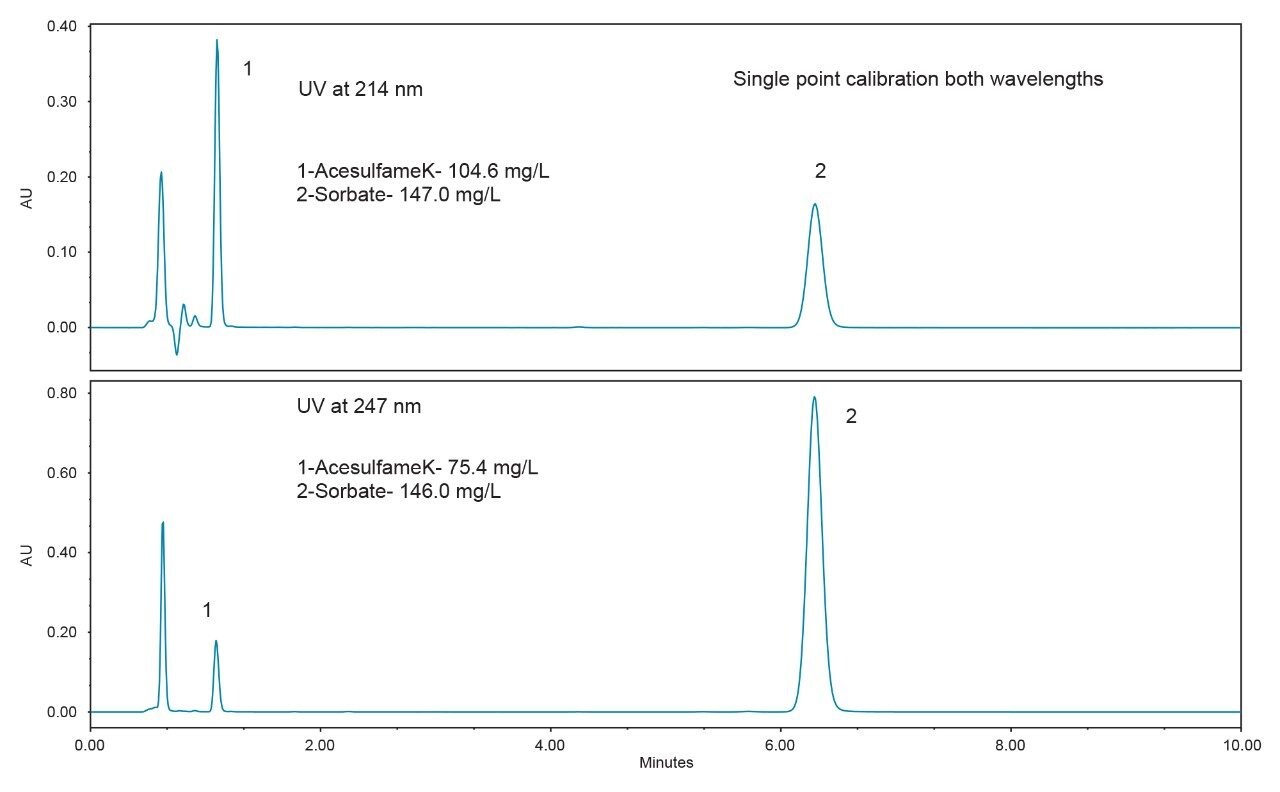
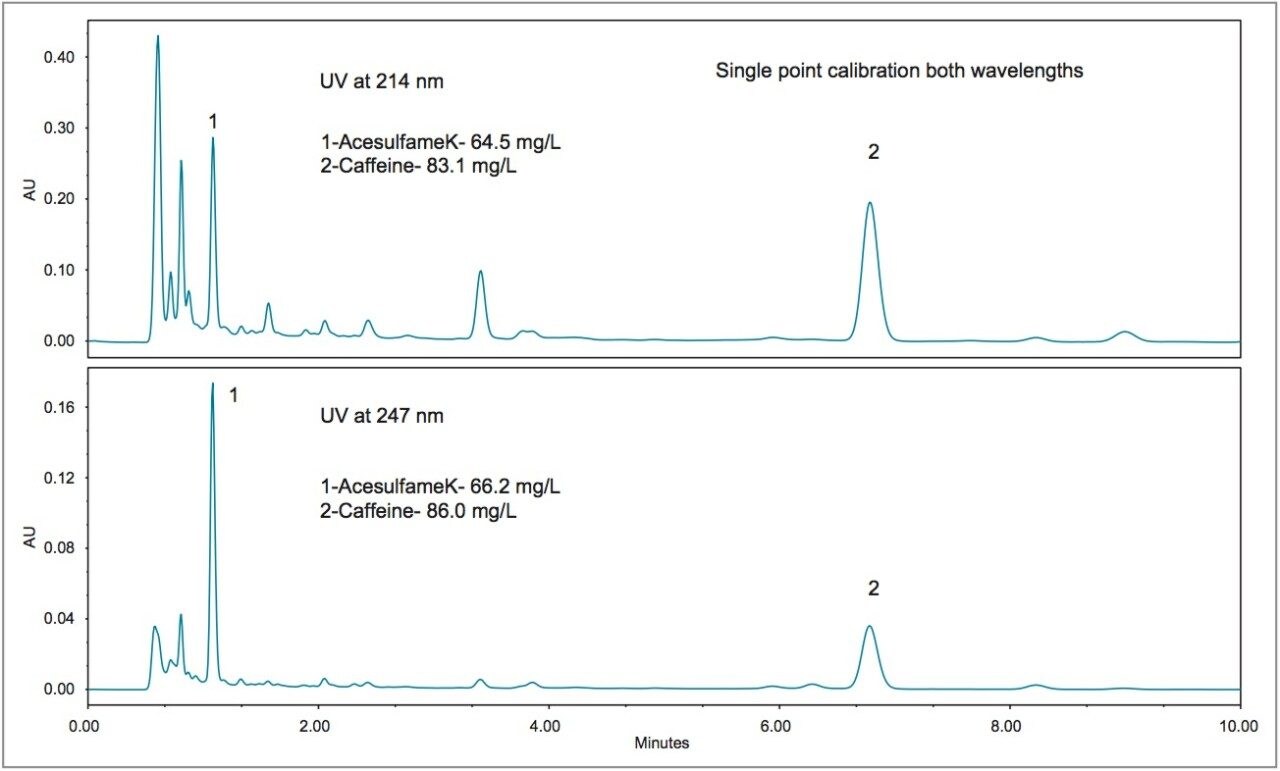
In order to assess the method with the sample types that are typically encountered, five different beverages were purchased from a local store for analysis. The resulting chromatograms from the analysis of these beverages are shown in Figures 3 to 7. The more traditional-style carbonated beverages are represented by two examples, a diet cola (Figure 3), and a lemonlime soft drink (Figure 4). For each sample, the amounts of each analyte were calculated using both wavelengths for comparison. In Figures 3 and 4, the calculated amounts using the two different wavelengths agree within 4% for all of the analytes. A single point calibration was used to calculate the amounts shown in Figures 3 to 7.
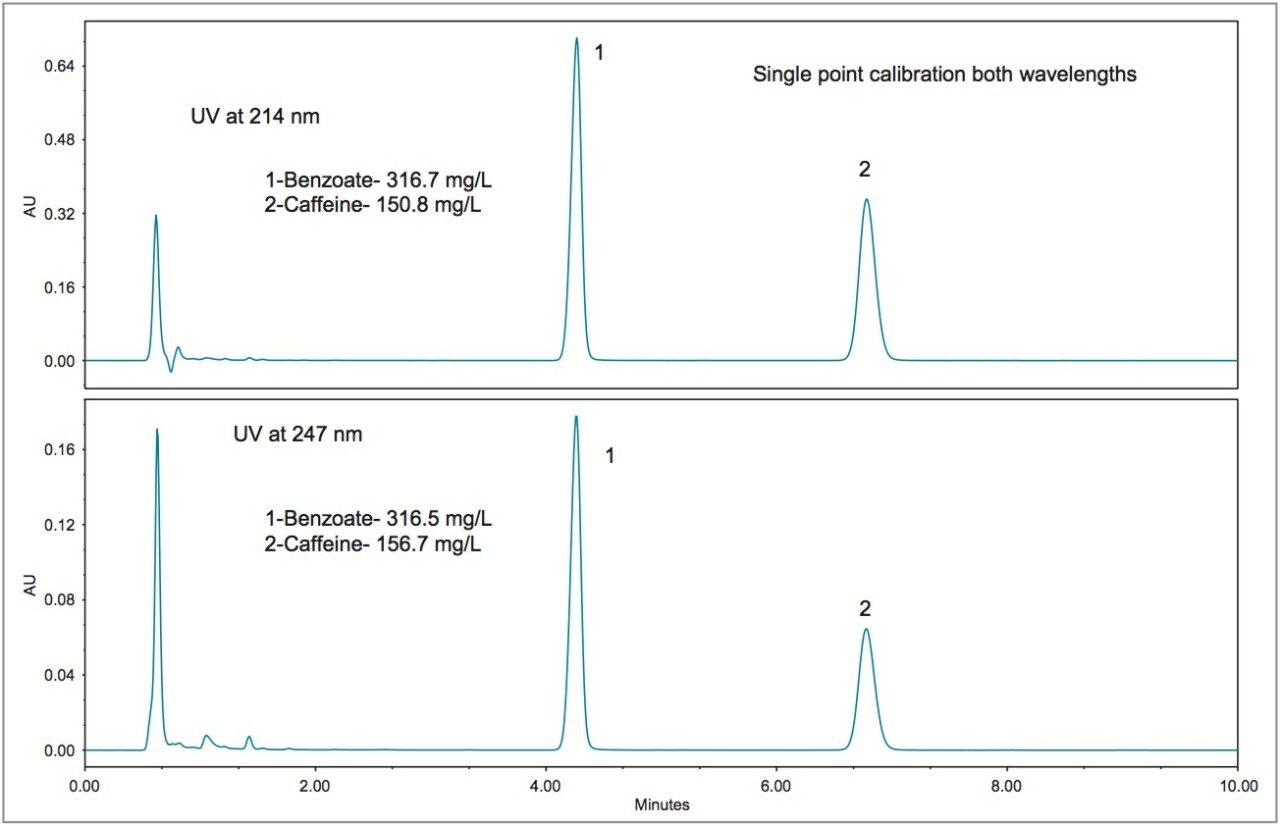
Newer formulations of soft drinks often include ingredients or additives that have known or perceived health benefits. Examples include essential nutrients, antioxidants, and plant extracts. Figures 5, 6, and 7 show three different examples. Figure 5 displays the chromatograms for a diet vitamin water, and Figure 6 for a diet energy drink. For both of these samples, the quantification at both wavelengths is in agreement (within 2.1%) with the exception of acesulfame K. The diet lemon tea showed excellent agreement at both wavelengths (within 3.4%), even for acesulfame K.
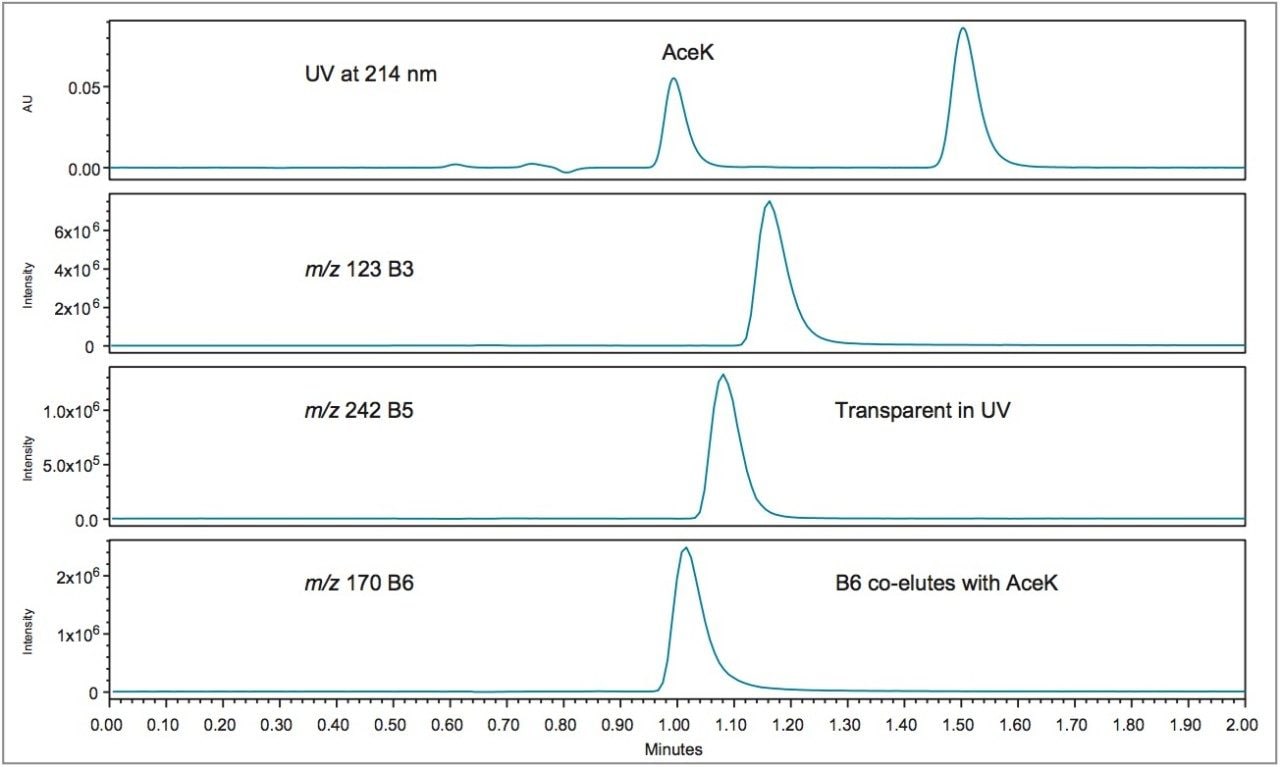
Further investigation of the difference in quantification for acesulfame K in the diet vitamin water and diet energy drink revealed that both of these beverages contain B vitamins, where the other soft drinks did not. Using the ACQUITY QDa Detector, which enables mass analysis of the analytes, it was apparent that the B vitamins closely elute with acesulfame K. Figure 8 shows an overlay of the vitamin water chromatograms of acesulfame K at 214 nm and the individual mass-to-charge ratios of the B vitamins acquired using the ACQUITY QDa Detector. Note that vitamin B6 coelutes with acesulfame K.
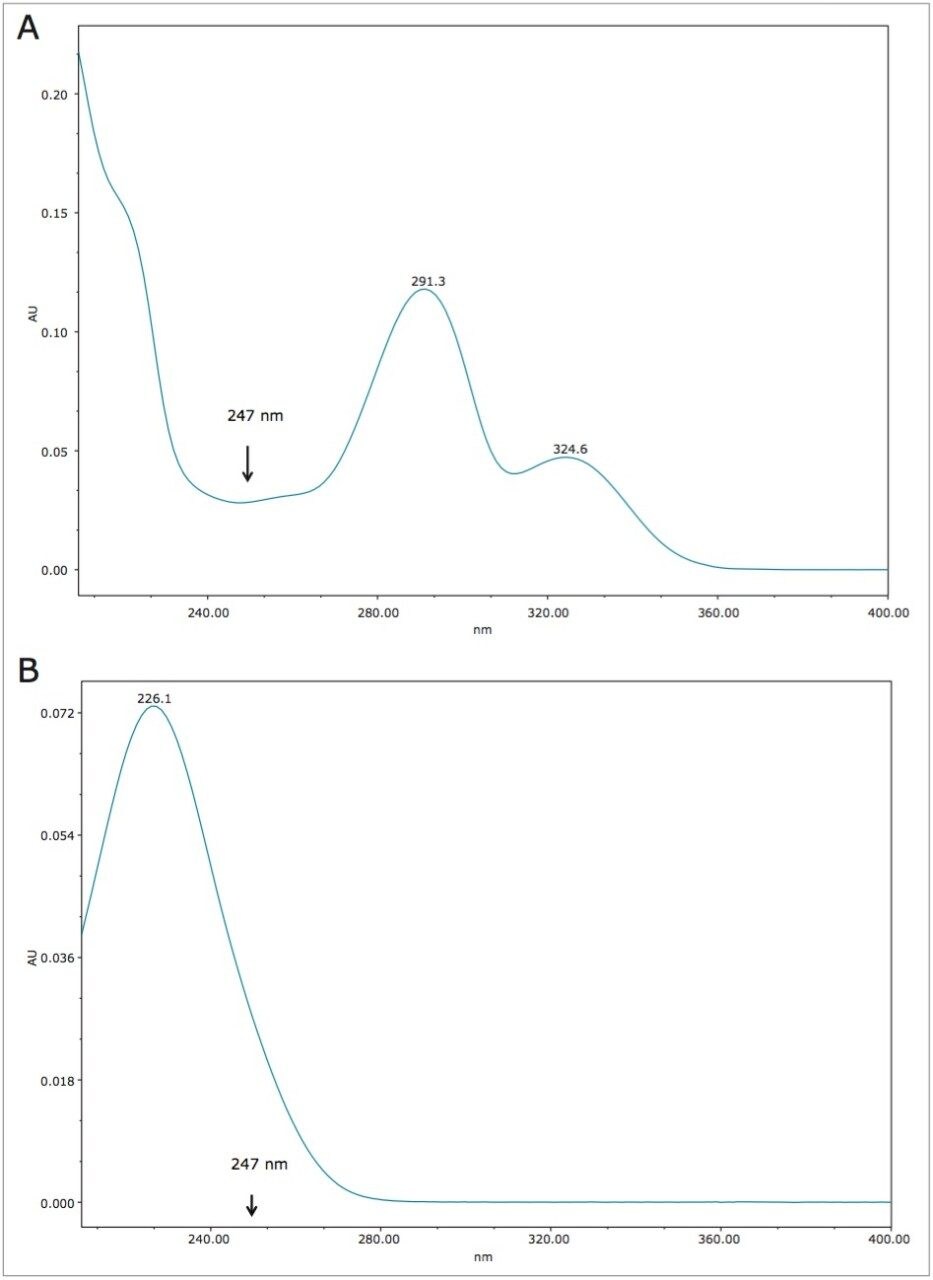
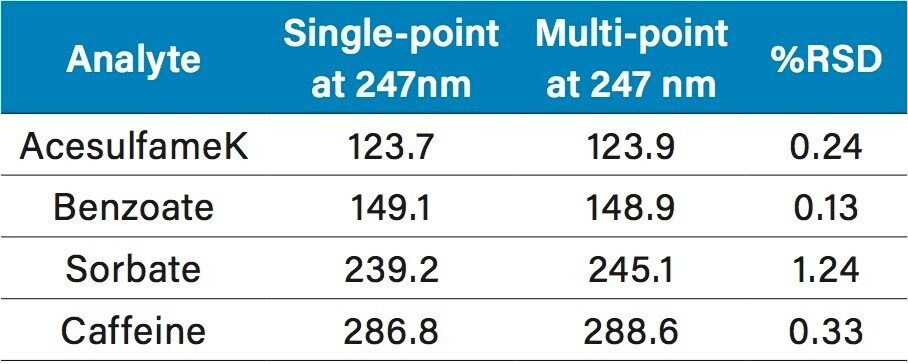
The UV spectra of vitamin B6 and acesulfame K are shown in Figures 9A and 9B, respectively. At 214 nm, which is non-specific, vitamin B6 has high absorbance and contributes to the overall response at 214 nm, along with acesulfame K. This results in over-estimation of the amount when using 214 nm. As can be seen, vitamin B6 is almost transparent at 247 nm, making this the wavelength of choice for beverages containing acesulfame K and vitamin B6.
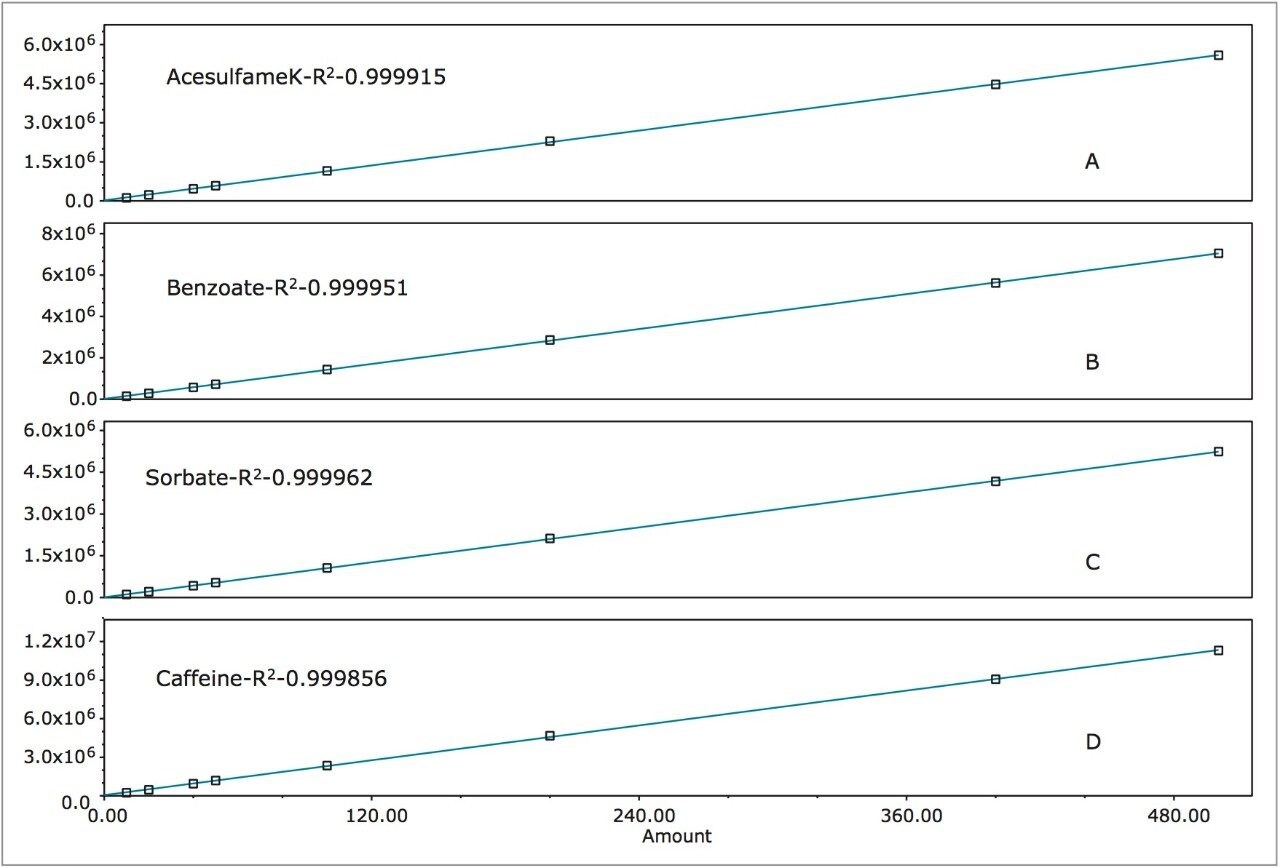
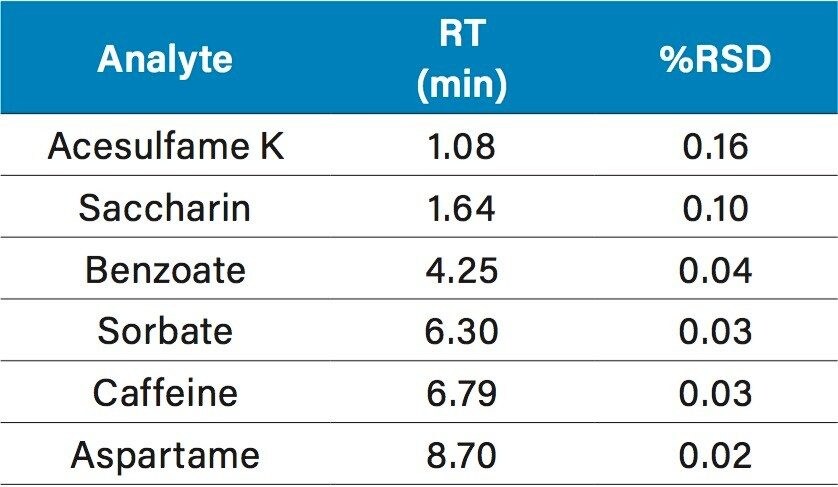
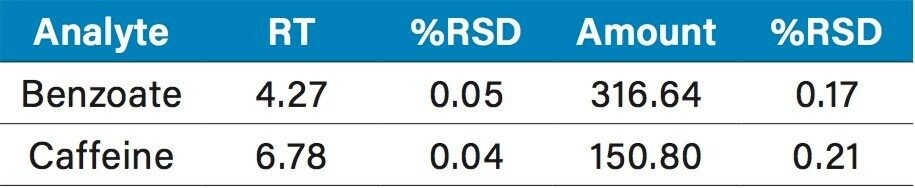
The calibration curves created using the multi-point calibration from the Waters Big-4 Standard are shown in Figure 10. R2 values were >0.999 for all compounds. Table 2 lists the retention time reproducibility for six injections of the Waters Beverage Analysis Standard. The %RSD was <0.2% for all analytes. Table 3 lists the reproducibility data for retention time and amounts for seven injections of the lemon-lime soft drink, which contained benzoate and caffeine. Quantification was made using the single-point calibration. Retention time reproducibility was 0.05% or less and the reproducibility for amount was <0.25%. Table 4 compares the quantification of the energy drink using both the single- and multi-point calibration at 247 nm to eliminate B vitamin interference.
Soft drink analysis using the ACQUITY Arc System provides a simple method for the analysis of soft drink additives. Implementation of such a procedure in a manufacturing environment has the capacity to improve overall workplace efficiency as well as:
720005474, March 2017