For research use only. Not for use in diagnostic procedures.
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates that the use of an online SPE system (ACQUITY UPLC Online SPE Manager) enables the automated, efficient analysis of mycophenolic acid by LC-MS for clinical research.
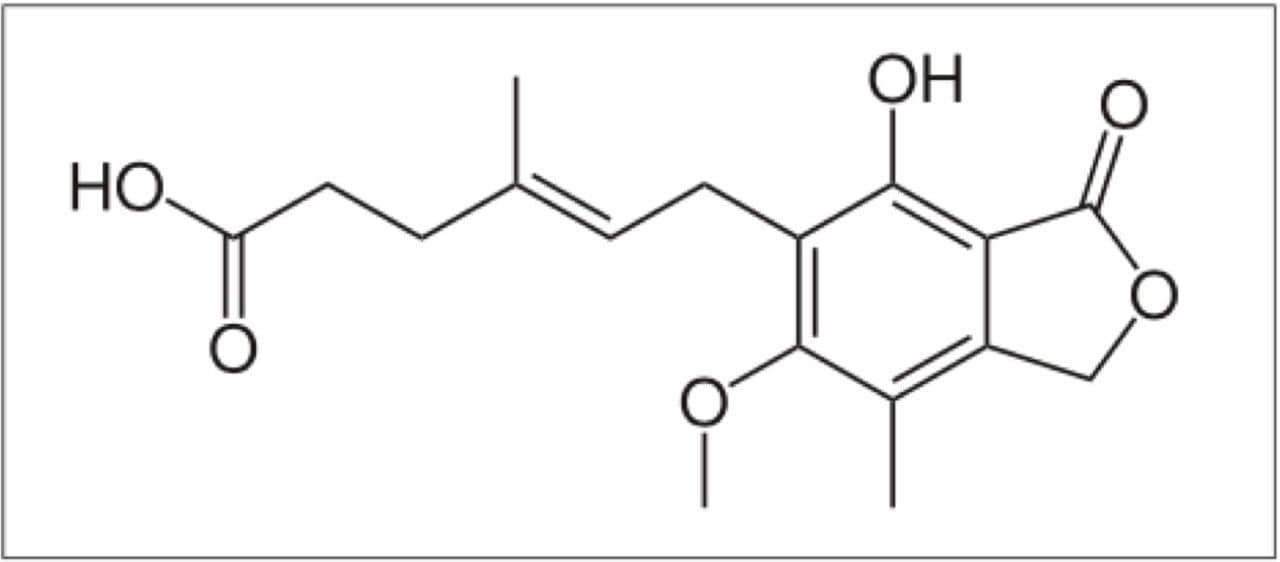
Mycophenolic acid (MPA), Figure 1, is an immunosuppresant drug that interferes with purine nucelotide synthesis required for the proliferative response of T and B Cells. Mycophenolic acid (MPA) based therapies are widely used in combination with calcineurin inhibitors as maintenance immunosuppression for kidney transplant recipients. Measurement of drug levels in plasma in clinical research can be performed by a variety of analytical methods including immunoassay, LC with UV detection, or LC-MS. LC-MS methods can provide a level of sensitivity and specificity for MPA analysis that is difficult of other methods to achieve.
However, the lack of availability of a simple MPA test that can be automated is a limitation to measuring this immunosuppressant.
In this application brief, an automated clinical research method for efficiently and accurately measuring MPA from plasma has been developed. This method takes advantage of the unique capabilities of an online SPE system (ACQUITY UPLC Online SPE Manager) to automate and integrate sample preparation with LC-MS analysis. This system enables the analytically sensitive and robust measurement of MPA in an automated fashion and compares favorably with existing research methods for analyzing MPA.
Plasma samples (50 μL) were added to 500 μL of deuterated internal standard MPA in 30% methanol containing 0.2 M zinc sulfate. Samples were then centrifuged to remove precipitated proteins, and the supernatants were removed for further analysis by LC-MS.
After protein removal, an aliquot of sample was injected into an LC-MS equipped with online SPE and analyzed.
Automated SPE was performed using a Waters MassTrak Online SPE Analyzer equipped with a XBridge C18 OSM Solid Phase Extraction (SPE) Cartridge.
Online SPE Cartridges were conditioned with 0.7 mL of methanol and equilibrated with 1 mL of water at flow rates of 3 mL/min. Sample (10 μL) was loaded onto the cartridge with water (0.1 mL) at a flow rate of 3 mL/min. Cartridges were then washed with 25% methanol (0.1 mL) at a flow rate of 3 mL/min. After washing, the cartridge was automatically transferred to the left clamp and eluted using the mobile-phase gradient for 1 min. The cartridge was then removed and the clamp flushed with water (0.1 mL) at a flow rate of 3 mL/min.
|
LC system: |
ACQUITY UPLC |
|
Column: |
ACQUITY UPLC HSS SB C18, 1.8 μm, 2.1 x 30 mm |
|
Column temp.: |
50 °C |
|
Flow rate: |
700 μl/min |
|
Mobile phase A: |
0.2 mM ammonium acetate with 0.1% formic acid in water |
|
Mobile phase B: |
0.2 mM ammonium acetate with 0.1% formic acid in methanol |
|
Gradient: |
Initial conditions 70/30 A/B |
Increase to 40% B over 0.75 mins. Then increase to 85% B over an additional 0.85 mins. Mobile phase B then increased stepwise to 98% for an additional 0.4 mins. Column was then re-equilbrated with initial conditions for 0.5 mins. The analysis time per sample was approximately 2.5 minutes injection to injection.
|
MS system: |
Xevo TQD |
|
Ionization mode: |
Positive |
|
Capillary voltage: |
0.5 kV |
|
Desolvation temp.: |
450 °C |
|
Desolvation gas: |
800 L/h |
|
Cone gas: |
25 L/h |

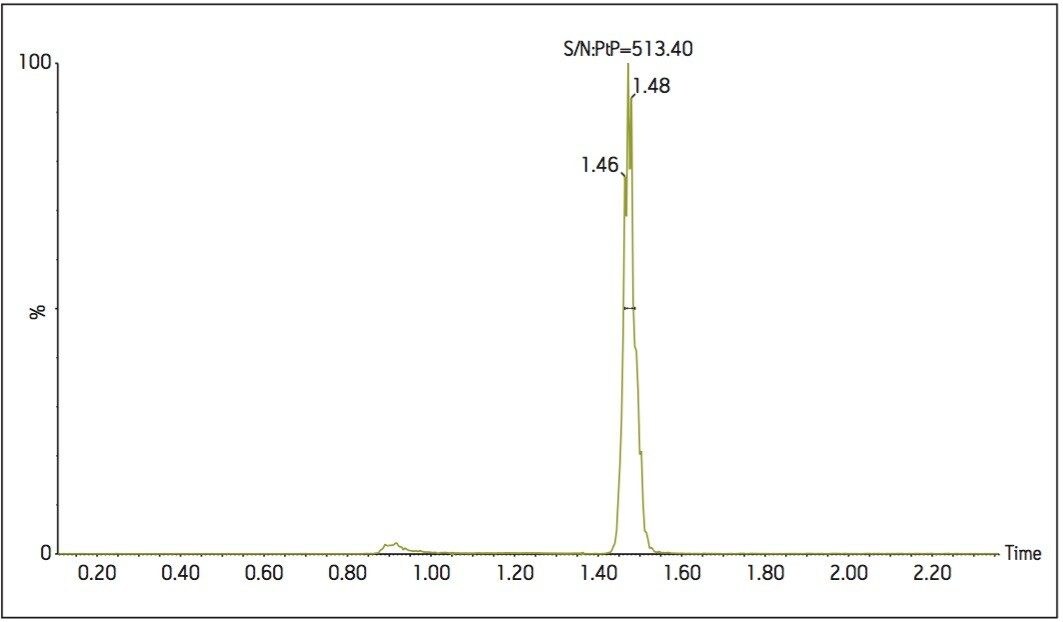
Mycophenolic acid and d3-mycophenolic acid eluted from the column at 1.47 min, as shown in Figure 2. The interfering peak at 0.9 min is caused by mycophenolic acid’s glucuronide metabolites. The glucuronide groups are labile and can fragment in the source of the mass spectrometer producing an isobaric interference. However, the peaks from the glucuronides are chromatographically resolved from mycophenolic acid, thus do not interfere with quantification. The total run time injection-to-injection was 3.0 min, and the overall time to prepare and analyze a 96-well plate of samples was approximately 5.5 hours.
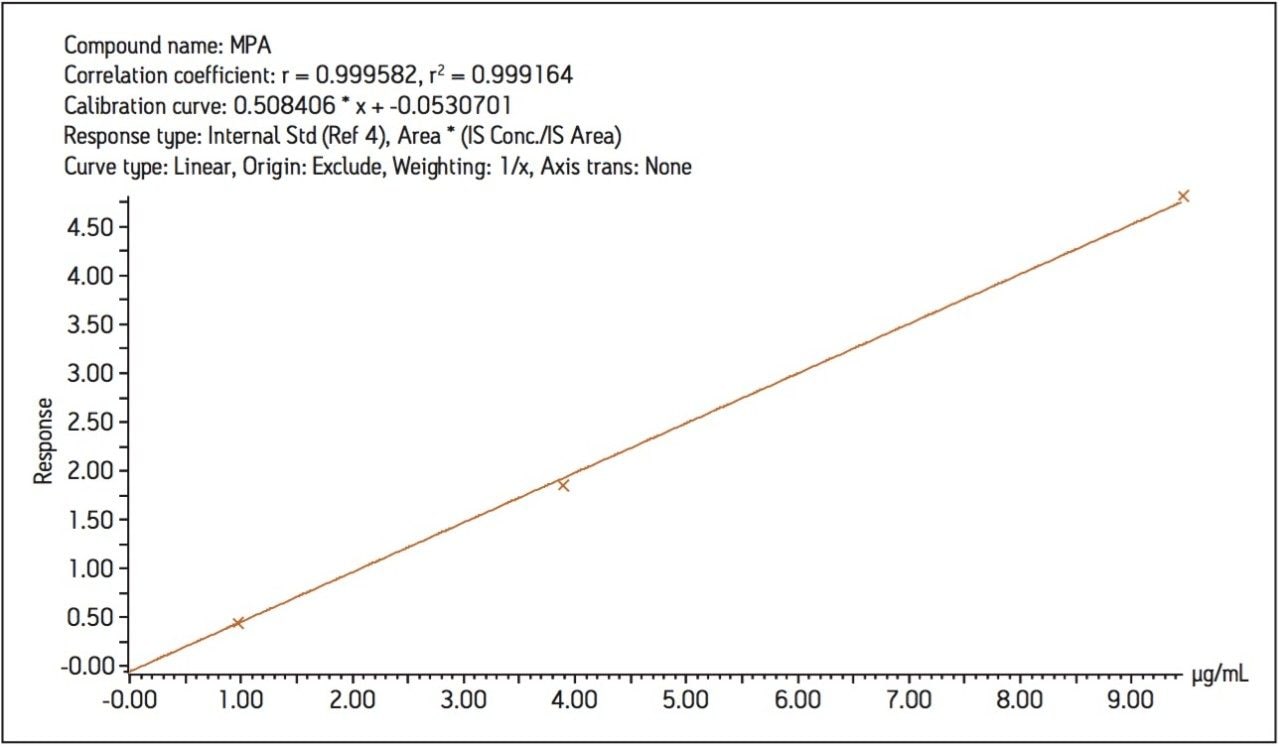
The method was found to be linear from 0.01 to 50 μg/mL MPA concentration as shown in Figure 3. The method LOD and LLOQ were found to be 2 ng/mL and 7 ng/mL respectively.
Inter-assay imprecision for low (1.94 μg/mL), mid (2.35 μg/mL), and high (5.50 μg/mL) concentration MPA QC samples were 5.8%, 8.4%, and 6.5%, respectively (n=25, days=5).
As part of the study, fifty authentic plasma samples from subjects receiving MPA were analyzed and the concentration of mycophenolic acid calculated. These values were compared to those obtained at a separate laboratory using a routine LC-MS method. Using Deming Regression, the agreement between the two data sets was described by the equation y=0.99x - 0.01, giving a bias of just 1% between the two methods, as shown in Figure 4. Bland Altman analysis also demonstrated good agreement of the methods with no systematic or proportional biases observed (data not shown).
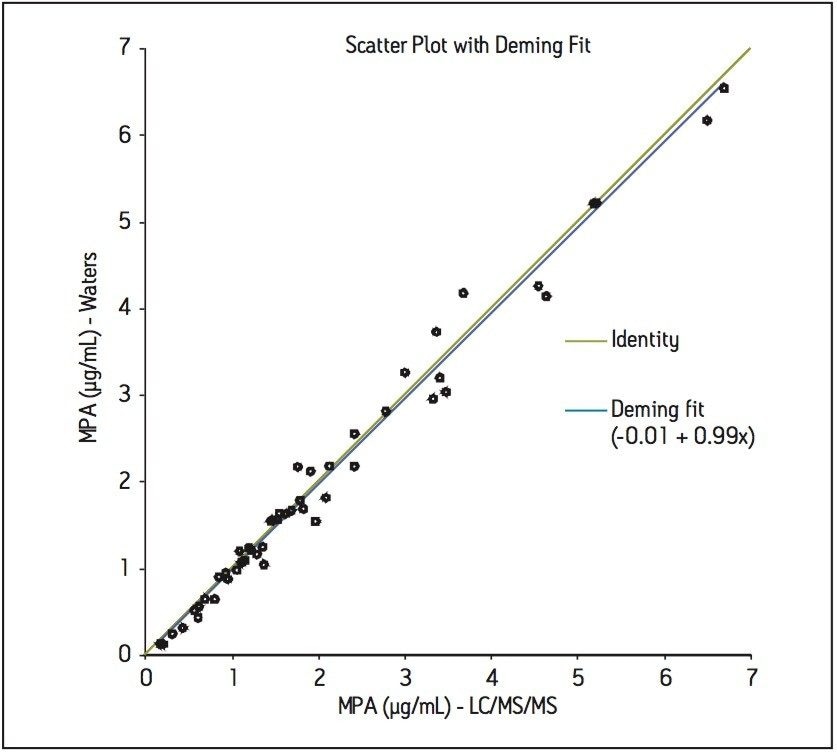
In this work, an automated LC-MS clinical research method has been developed for the measurement of MPA from plasma. The method utilizes LC-MS equipped with an online SPE system. This combination of SPE sample preparation coupled with the analytical power of LC-MS can prove a very analytically sensitive and efficient method for the analysis of this important immunosupressant.
The method developed here provides:
720005126, August 2014