
This is an Application Brief and does not contain a detailed Experimental section.
This application brief illustrates the utility of mass-directed purification on preparative chromatography systems configured with an ACQUITY QDa Detector.
Mass-directed purification with an ACQUITY QDa Detector configured in the system selectively isolates compounds of interest from complex mixtures and reduces the number of fractions for subsequent evaporation and analysis, leading to a more efficient workflow.
Although traditional compound isolation is usually performed with UV detection, introducing a mass detector to the preparative chromatography system provides an increased level of confidence in elucidating target compounds and collecting them. With the specificity that mass detection brings to purification, fewer fractions are collected (reducing evaporation time and analysis), compounds without chromophores are more easily targeted, and, when combined with UV detection, higher product purity and yield may be realized. Streamlining the purification process ultimately leads to improved efficiency with both time and cost savings. In this application brief, we illustrate how mass-directed purification with an ACQUITY QDa Detector configured in the Waters AutoPurification System decreases postcollection fraction analysis and workup.
The ACQUITY QDa Detector, with its small size, automatic calibration and optimization routines, and easy system integration, makes mass-directed isolation more readily accessible to preparative chromatographers.
While the isolation of compounds from sample mixtures can be accomplished with UV detection, mass-directed isolation effectively simplifies the purification process by only targeting specific compounds whose masses are known. As shown in Figure 1, the UV-directed collection is nonspecific and all compounds are collected because the collection threshold is satisfied in the fraction method. Without prior knowledge of the elution order, the mass-directed purification identifies all of the compounds and yields only two fractions, those corresponding to the two specific masses requested by the user. With only the desired compounds collected, valuable space is saved in the fraction collection bed, fewer fractions require evaporation and analysis, and the overall purification process efficiency is improved. Faster turnaround time leads to increased productivity with savings in both time and cost. Figure 2 shows the total ion chromatogram and the extracted ion chromatograms for each of the two desired drug compounds, with the triggering mass as well as the tube location in the fraction collector bed.
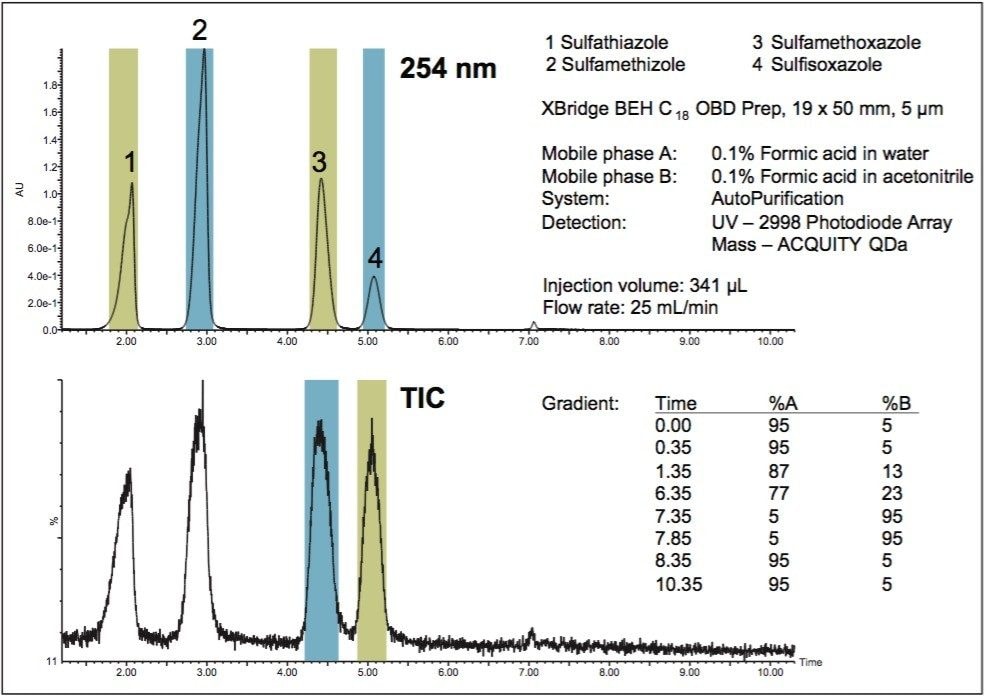
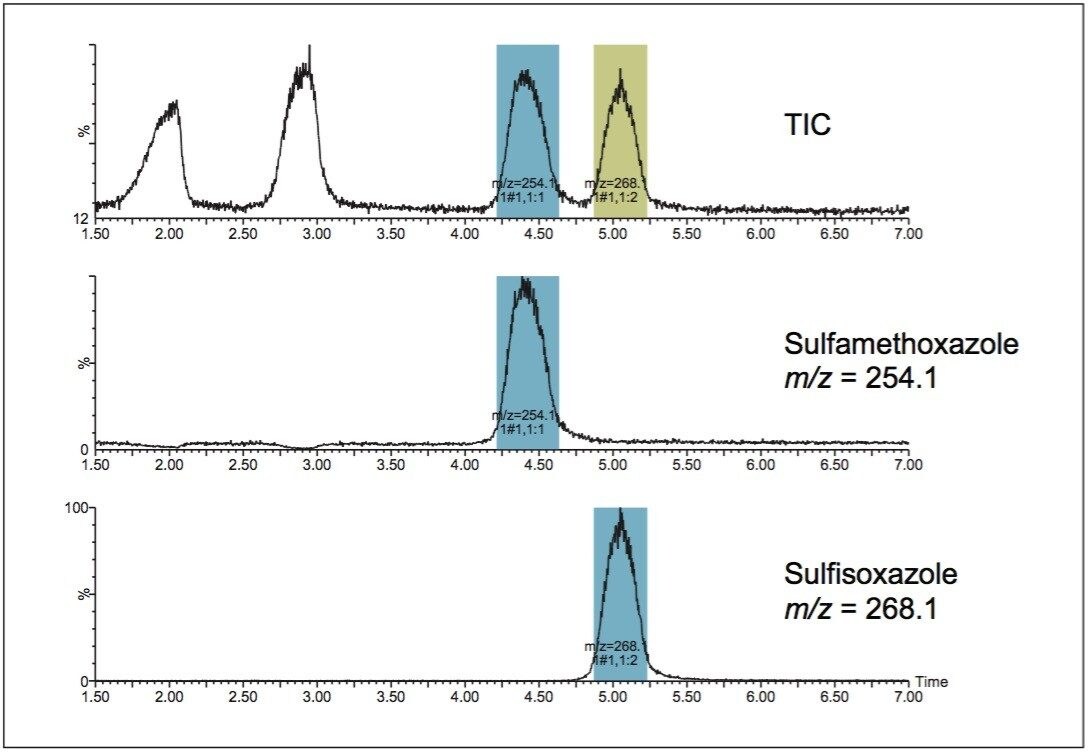
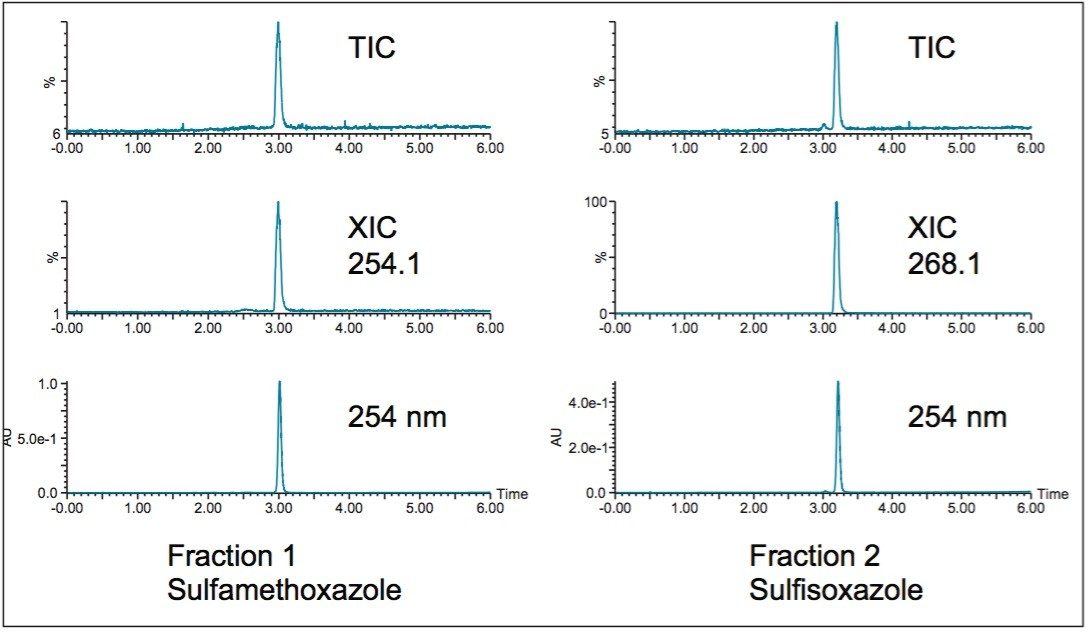
Fraction analysis with injections drawn directly from the collection tubes before evaporation is shown in Figure 3. UV and mass analysis indicate that the target compounds are pure.
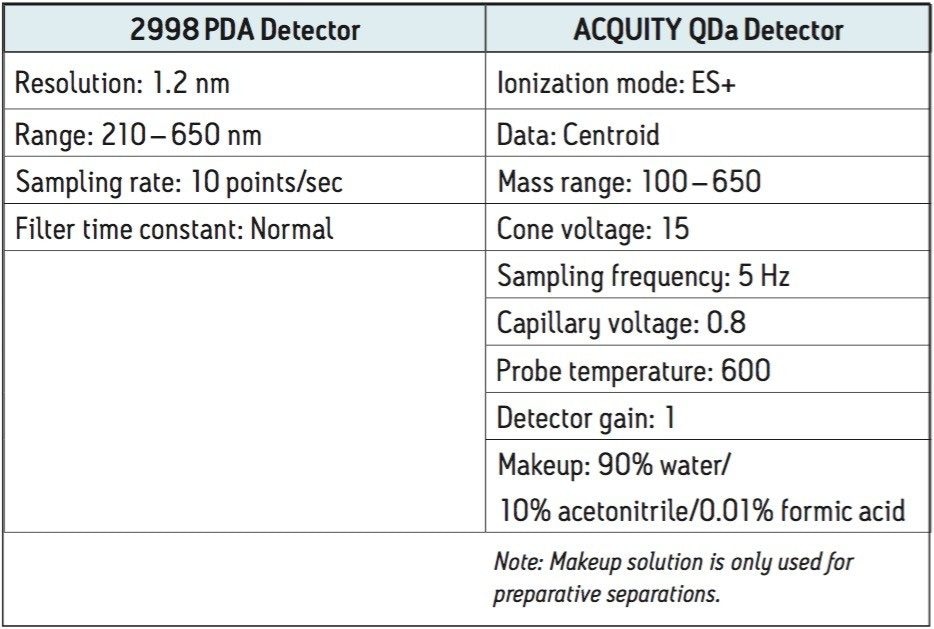
Both the analytical and preparative chromatographic methods utilize many common UV and mass spec parameters. These are listed in Figure 4.
720005107, July 2014