This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates a fast, easy, and cost-effective method for the enantiomeric separation of nebivolol isomers using UltraPerformance Convergence Chromatography (UPC2).
UPC2 separation of nebivolol allows for up to a 65% reduction in analysis cost and up to 80% improvement in analysis throughput compared to traditional LC methods.
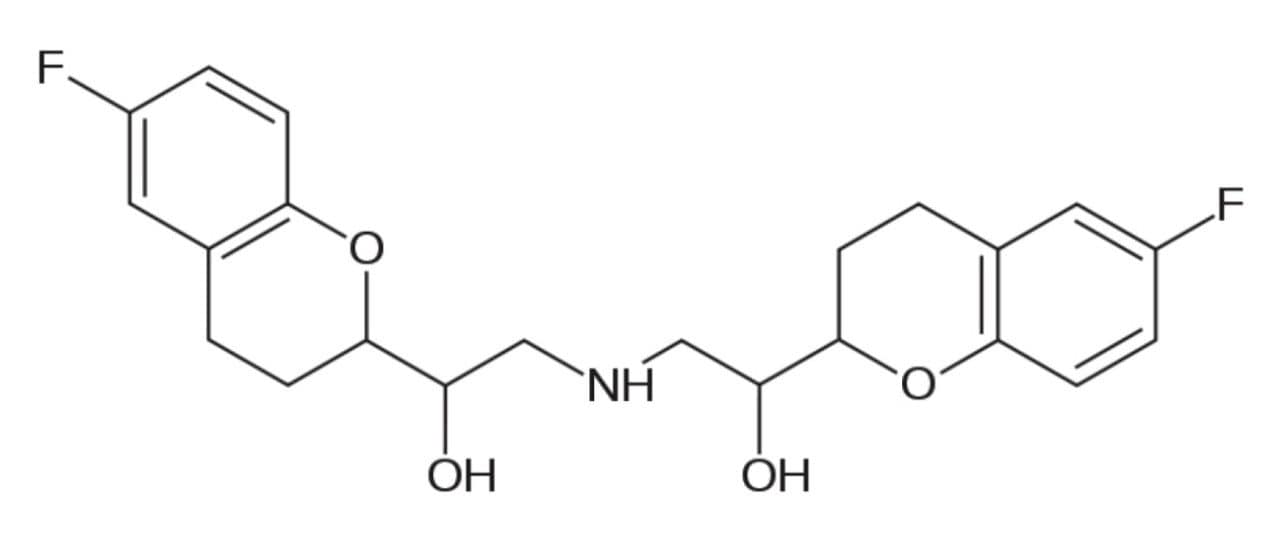
Nebivolol (Figure 1) is a beta blocker drug with nitric oxide-potentiating vasodilatory effects used in the treatment of hypertension. Beta blockers are one of the most highly recommended and prescribed drugs for the treatment of hypertension and heart diseases throughout the world. Studies show that the D-isomer of nebivolol is primarily responsible for beta selectivity, whereas the L-isomer potentially provides the anti-hypertensive effect of the D-isomer. Enantiomeric profiling is often needed to monitor the metabolic pathways for both isomers.
Chiral method development for these types of compounds is often time-consuming, since screening of multiple chiral stationary phases is required. The analysis and column regeneration times for chiral stationary phases using normal-phase solvents is often very high. The solvents used for traditional normal-phase chromatography such as n-hexane, halogenated solvents, etc., are toxic and costly in terms of both use and disposal.
Chiral analysis is typically performed in the early stage of drug discovery. Due to its superior resolving power and high speed, convergence chromatography proves to be a powerful analytical technique in chiral analysis. Use of convergence chromatography also eliminates the use of toxic solvents typically associated with normal-phase LC chiral analysis.
The Waters ACQUITY UPC2 System uses supercritical CO2 as the primary mobile phase component. UPC2 technology applies the performance advantages of UPLC to supercritical fluid chromatography (SFC). UPC2 allows for screening and method development for the enantiomeric separation of pharmaceutical compounds within a very short time, as well as for reduced solvent consumption.
This study demonstrates a fast and effective method for the enantiomeric separation of the isomers of nebivolol using UPC2.
For the method demonstrated here, a nebivolol standard was dissolved in 100% methanol at a concentration of 500 ppm. The ACQUITY UPC2 System was used with a 4.6 x 150 mm, 3-μm chiral column, and an ACQUITY UPC2 PDA Detector at 220-nm wavelength.
Using the method described above, the isomers of nebivolol have a USP resolution of 2.1.
The results obtained are highly reproducible over several consecutive injections. The %RSD value for retention time and area for 20 replicate injections were 0.1 and 1.0, respectively.
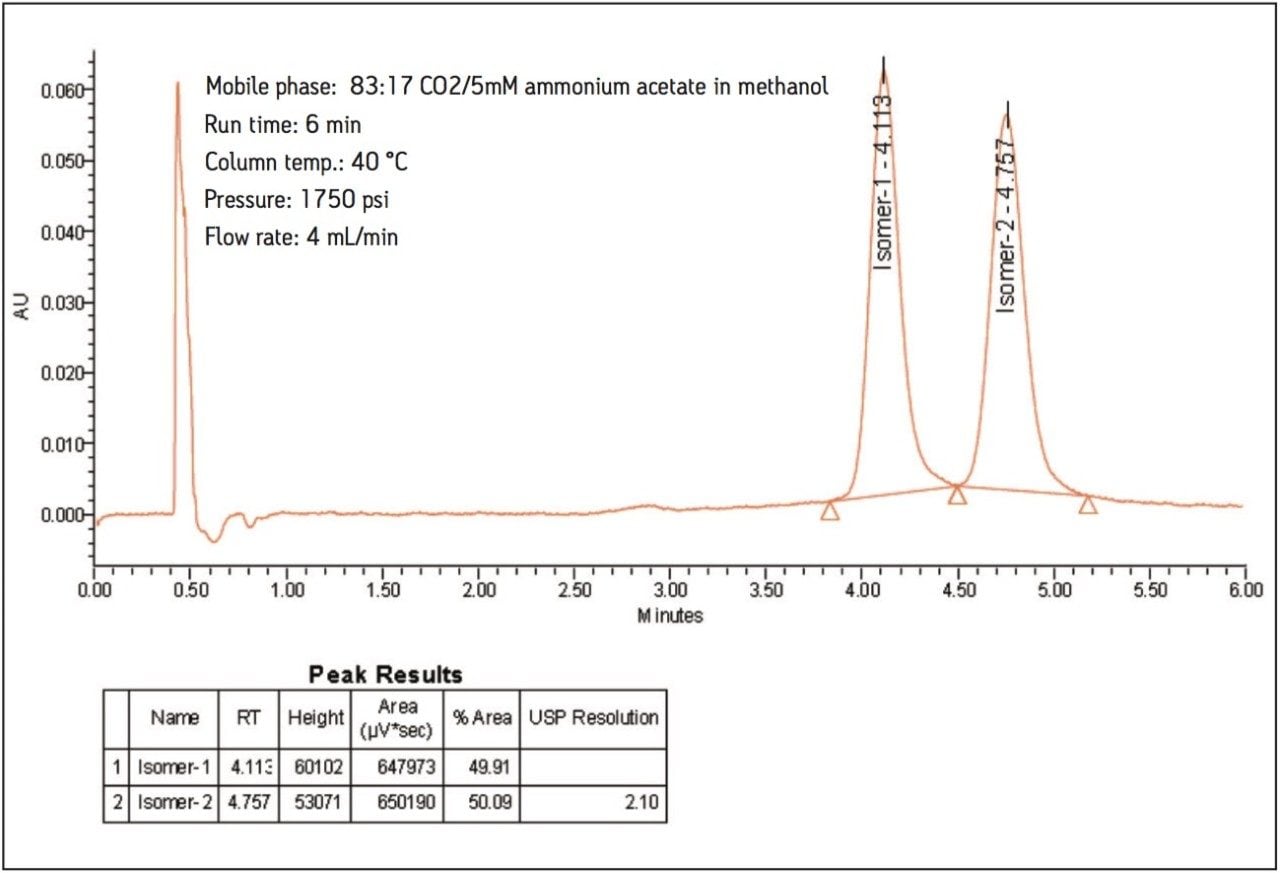
The ACQUITY UPC2 System with a 3-μm column was used to demonstrate an easy and effective method for the enantiomeric separation of nebivolol. The isomers of nebivolol have been separated to base line within 6 minutes. Using the method demonstrated here, up to a 65% reduction in analysis cost and up to 80% improvement in analysis throughput is possible compared to traditional LC methods. At the same time, where some of the current HPLC normal-phase method for nebivolol has recommended column conditioning time of 12 to 15 hours before starting the analysis, 5 to 10 minutes column conditioning is sufficient in UPC2 when using the method described here. The method is highly reproducible over several injections. The mobile phase used is compatible with a mass spectrometer, thus extending the applicability of UPC2 to bioanalytical studies.
720004902, March 2014