
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the use of qualitative mass spectral data in method development, using an ACQUITY UPLC H-Class System with ACQUITY QDa Detector to confirm the identity of ziprasidone HCl and related compounds.
The ACQUITY QDa Detector aids in the development of efficient and robust screening methods by minimizing the need for standard runs to confirm the identity of peaks by retention time.
Method development typically involves screening chromatographic parameters such as columns, organic solvents, buffers, gradient slope, flow rate, temperature, and so on. Any of these parameters may be modified to alter the resolution to achieve the required analytical quality.
Small modifications in pH often alter the relative retention (elution position) of compounds in a reversed-phase separation. As these separation variables are investigated, it is essential to track changes in chromatographic behavior for each of the sample components. At the same time, recognition of coeluting species is required.
Without accurate and complete peak tracking, development times can be prolonged and significant impurities may be unrecognized. In addition, incorrect identification or failure to identify impurities may compromise the safety and efficacy of the end pharmaceutical product. Utilizing a mass detector enables the analytical laboratory to correctly monitor peak retention by mass spectrometric detection.
In this study, we take advantage of qualitative mass spectral data acquired using an ACQUITY QDa Detector to track the elution of ziprasidone HCl and its USP-specified related compounds over a series of different mobile phase pH experiments. This method development process was also facilitated by using the ACQUITY UPLC H-Class with Auto•Blend Plus Technology to control pH.
Auto•Blend Plus, which is included with the ACQUITY UPLC H-Class System, was used to program the blending of acid and base stock buffers with organic and aqueous solvents to deliver a mobile phase with a constant pH. The ACQUITY QDa Detector was used to confirm identity of the ziprasidone HCl and related compounds.
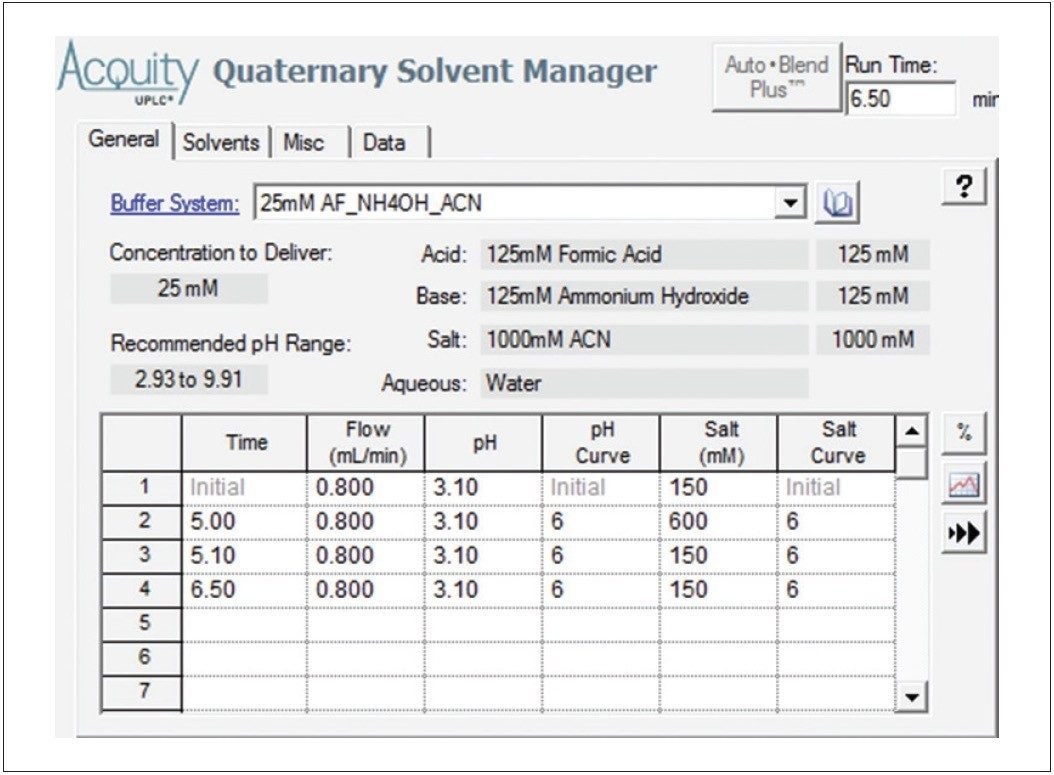
In this pH screening study, 125 mM formic acid and ammonium hydroxide stock solutions, acetonitrile, and water were programmed for mixing by the quaternary pump to deliver mobile phases with pHs of 3.1, 4.0, and 5.0. The Auto•Blend Plus method, set at pH 3.1, is shown in Table 1. The impact of pH on the separation of ziprasidone HCl and the related compounds is displayed in Figure 1.
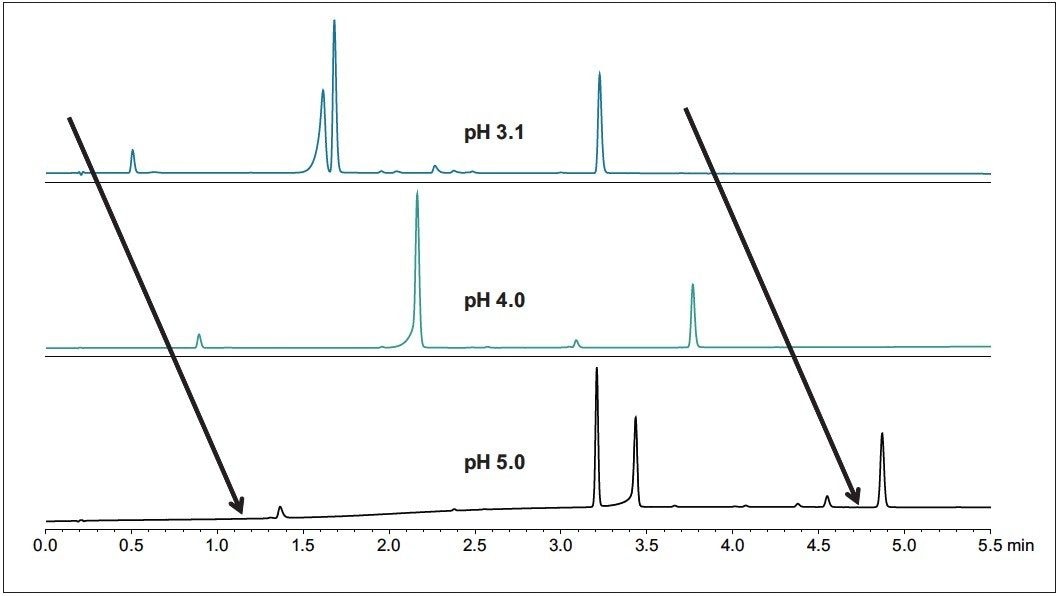
As shown in Figure 1, an increase in pH results in a higher retention of all the peaks. Fewer peaks were observed with pH 4.0 mobile phase than with 3.1 or 5.0. Tracking and identification of the peaks over the method developments runs with different pHs was performed using an ACQUITY QDa Detector.
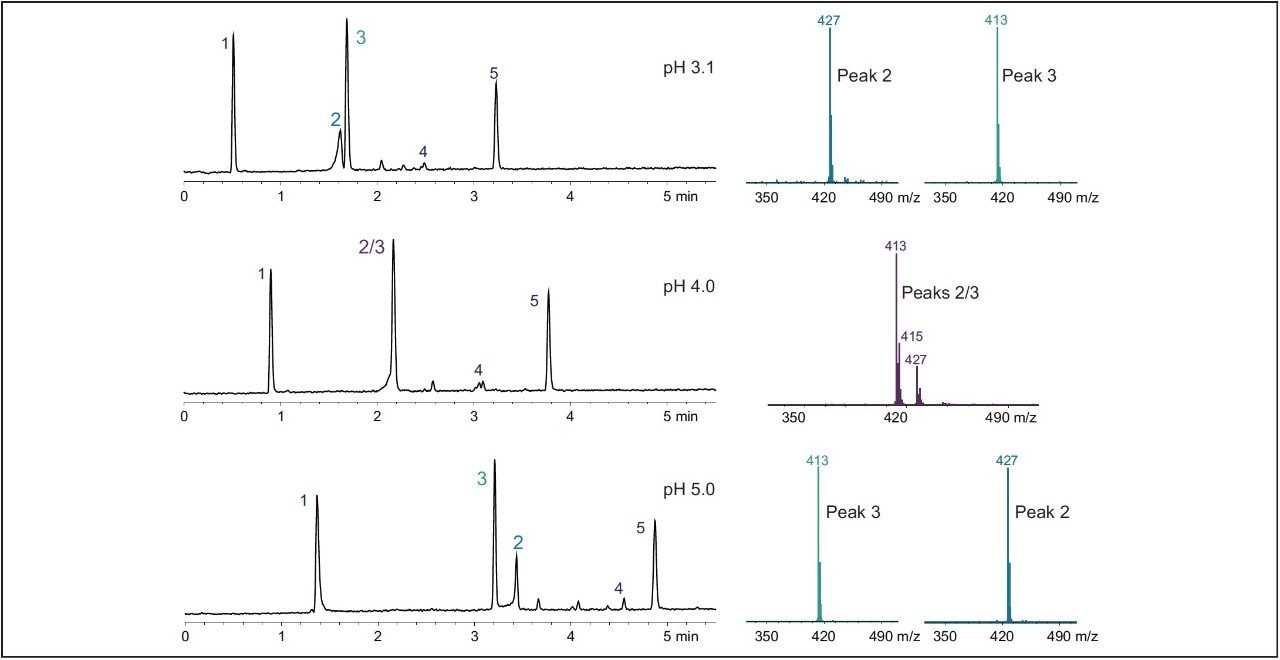
Tracking the elution of the peaks by mass detection is displayed in Figure 2. The mass spectra analysis confirmed the identity of the peaks and complemented tracking the elution order of peak 2 with the UV data.
In summary, the ACQUITY QDa Detector is a synergistic element of the chromatographic system that provides mass spectral molecular information for analytical scientists in a quick manner, without the need for high-end mass spectrometry. It streamlines development of efficient and robust methods by minimizing the need for standard runs to confirm the identity of peaks by retention times.
When used in conjunction with Empower 3 Software, which integrates optical and mass data processing, the mass spectral data can be interrogated in the same workflow as the ACQUITY UPLC PDA Detector data.
The ACQUITY QDa Detector was used to track sample components during development of the UPLC method for the separation of Ziprasidone HCl and its USP-specified impurities. The ACQUITY QDa Detector was designed to complement the optical data with the enhanced qualitative mass spectral data to confirm the identity of components using an orthogonal detection technique.
Overall, the ACQUITY QDa Detector coupled with the ACQUITY UPLC H-Class System and and Auto•Blend Plus Technology provides complete and rapid chromatographic separation and characterization of compounds, streamlining a laboratory’s workflow in the analysis of pharmaceutical products.
720004801, October 2013