In this application note, we report the development of a sensitive LC-MS/MS assay for the analysis of the signature peptide of trastuzumab with an assay sensitivity of 5 nM trastuzumab (0.75 μg/mL) in human serum.
This work demonstrates the capability of Xevo TQD to conduct bioanalysis of large protein molecules, as trastuzumab, a monoclonal antibody. Oasis micro-elution plates, the ACQUITY UPLC H-Class System, and an advanced tandem quadrupole mass spectrometer (Xevo TQD) were used for the development of a sensitive method for the quantification of trastuzumab in human serum digest. This application note addresses some critical challenges in the world of bioanalysis, such as developing a method to quantify protein molecules with desired sensitivity in a robust, reproducible, high-throughput LC-MS/MS system.
Monoclonal antibodies (mAbs) comprise a new group of therapeutic compounds, which are used to treat cancer, auto-immune diseases, and systemic infections.1 HER2/neu receptor is over-expressed in about 25% of all breast cancer patients.2,3 Trastuzumab, as shown in Figure 1, directed against the HER2/neu receptor, is primarily used to treat certain breast cancers. It selectively binds with high affinity to the extracellular domain of the human epidermal growth factor receptor 2 protein, HER2. Trastuzumab is produced by recombinant DNA technology in a mammalian cell (Chinese hamster ovary) culture containing the gentamicin antibiotic. Trastuzumab is administered by intravenous infusions of 10 to 500 mg once every week. The compound demonstrated dose-dependent pharmacokinetics, with an average half-life of two and 12 days at the 10- and 500-mg dose levels, respectively. The volume of distribution of trastuzumab was approximately that of serum volume (44 mL/kg). At the highest weekly dose studied (500 mg), mean peak serum concentrations were 377 μg/mL.4
Mass spectrometry is the analytical tool of choice for qualitative identification of mAbs. Quantification of mAbs in biological matrices utilizes enzyme-linked immune sorbent assay (ELISA), the most widely applied technique. However, LC-MS exhibits better specificity, and generates a better protein-specific charge envelope. In addition, structural changes in the molecule can be detected by the mass spectrometer, which is helpful in obtaining more insight in degradation patterns of mAbs, or to detect post-translational modifications. ELISA generates a result based on antibody binding properties, and does not reveal changes in the structure of mAbs. In this application note, we report the development of a sensitive LC-MS/MS assay for the analysis of the signature peptide of trastuzumab with an assay sensitivity of 5 nM trastuzumab (0.75 μg/mL) in human serum.
A stock solution of trastuzumab (a 150 kDa monoclonal antibody) was spiked with the internal standard (the 13C15N-isotopically labeled extended peptide GRFTISADTSK) and digested with trypsin to produce a stock solution containing 5 μM digested trastuzumab and 5 μM internal standard peptide (FTISADTSK). In parallel, 400 μL of human serum was dispensed in 10 Eppendorf vials (40-μL serum/vial) and digested with trypsin following the same procedure, to produce 400 uL of human serum digest in each vial. The digestion protocol involved sample denaturation (with 0.05% RapiGest at 80 °C for 10 min), disulfide bond reduction (in the presence of 20 mM dithiothreitol (DTT) at 60 °C for 60 min), cysteine alkylation (with 10 mM iodoacetamide (IAM) at room temperature for 30 min in the dark), and overnight digestion with porcine trypsin (25:1 (w/w) protein/ enzyme). Following digestion, 100 μL of digested peptides were diluted 1:1 with a solution containing 4% H3PO4 and loaded onto an Oasis MCX mixed-mode μElution plate (P/N 186001830BA). Digests were washed with 200 μL of 2% FA and 200 μL of 5% methanol before being eluted with 2 x 50 μL aliquots of 25% ACN in 2% NH4OH (pH 10). The trastuzumab digest was spiked into the human serum digest (using more than 90% of the serum digest matrix for each dilution) at the following concentrations: 5, 10, 20, 50, 100, 200, and 500 nM trastuzumab.
The analysis was performed on an ACQUITY UPLC H-Class System. A 10-μL aliquot of the sample was injected onto an ACQUITY BEH130 C18 2.1 x 150 mm, 1.7 μm column. The column temperature was maintained at 35 °C, with a flow rate of 0.3 mL/min. Mobile phases contained 0.1 % (v/v) formic acid (FA) in water (A), and 0.1% (v/v) FA in acetonitrile (B). Peptides were eluted with a linear gradient from 0% to 30% B over 10 min. The column effluent was monitored using a Xevo TQD Mass Spectrometer operated in selected reaction monitoring (SRM) positive ion electrospray mode. Four SRM transitions were monitored continuously throughout the LC-SRM assay using a dwell time of 40 ms: two SRMs monitored the endogenous signature peptide FTISADTSK from trastuzumab (485.2 → 721.4 for peptide quantification; 485.2 → 608.3 for peptide confirmation), while the other two SRM channels monitored the corresponding 13C15N-isotopically labeled internal standard peptide FTISADTSK (489.2 → 729.4 for peptide quantification; 489.2 → 616.3 for peptide confirmation).
Trastuzumab signature peptide (FTISADTSK) eluted with a retention time of 7.55 minutes, as shown in Figure 2. The data illustrates the injection of a 5 nM trastuzumab standard spiked in the human serum digest immediately following the analysis of human serum digest blank. This data shows very symmetrical peaks produced by the chromatography system that have a width at the base of approximately 4 seconds. The lower limit of quantification (LLOQ) for the assay was determined to be 5 nM (or 0.75 μg/mL) of trastuzumab in human serum, with an RMS signal to noise ratio of 27:1. A typical calibration obtained for the assay for the FTISADTSK signature peptide of trastuzumab is shown in Figure 3, with a correlation coefficient of 0.99326 using a 1/x weighting linear regression.
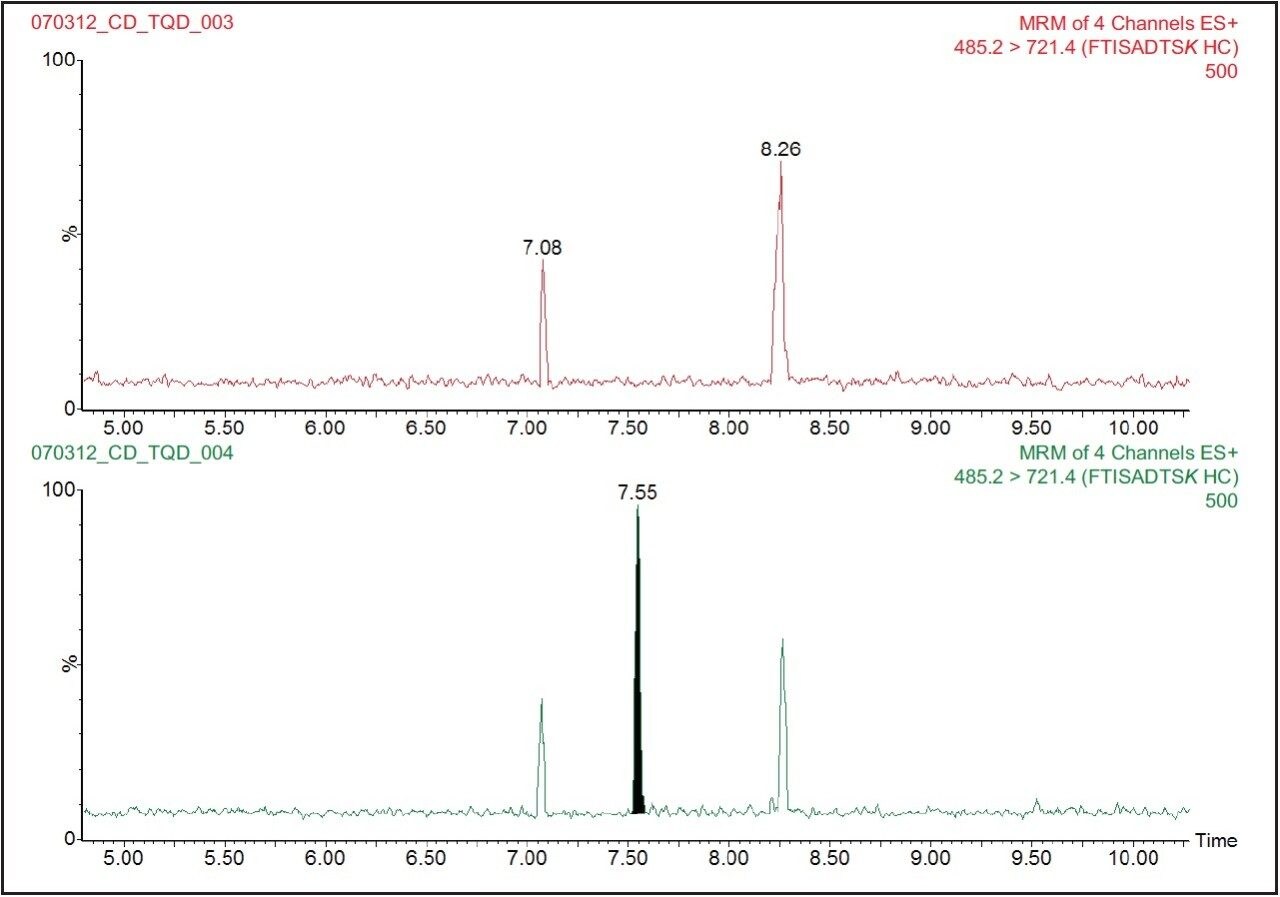
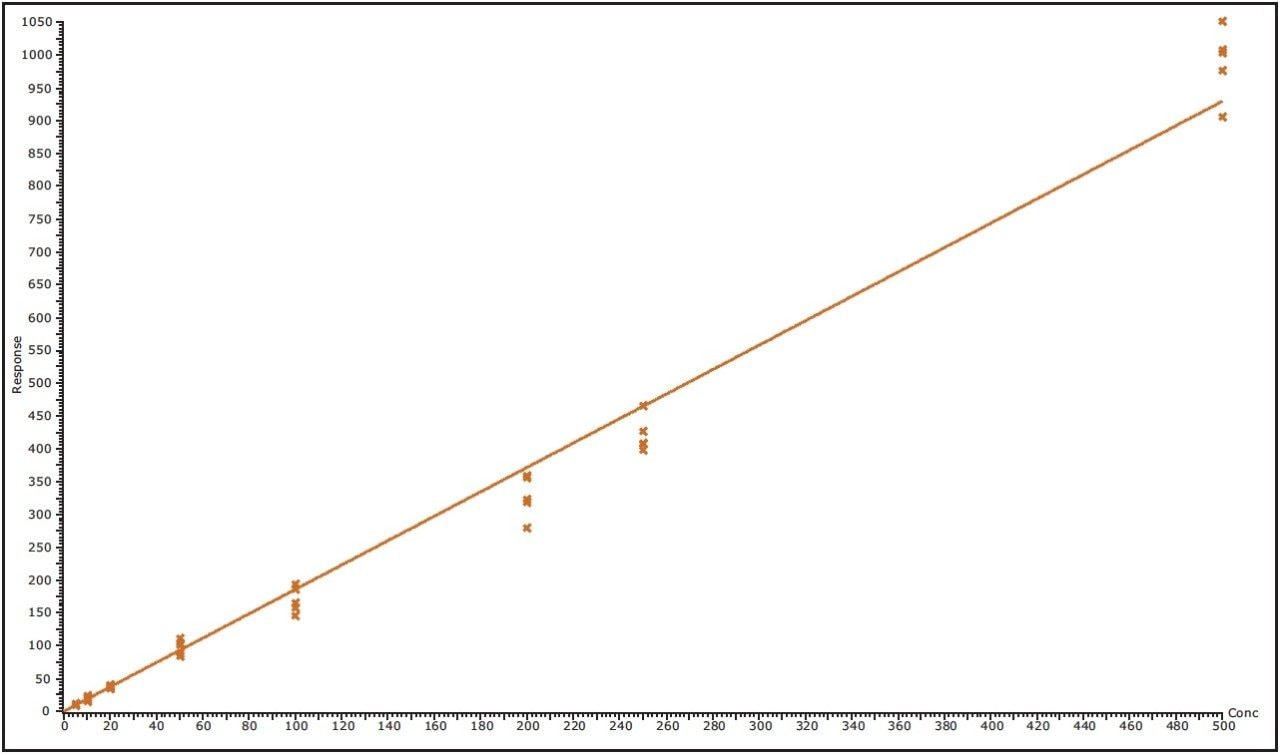
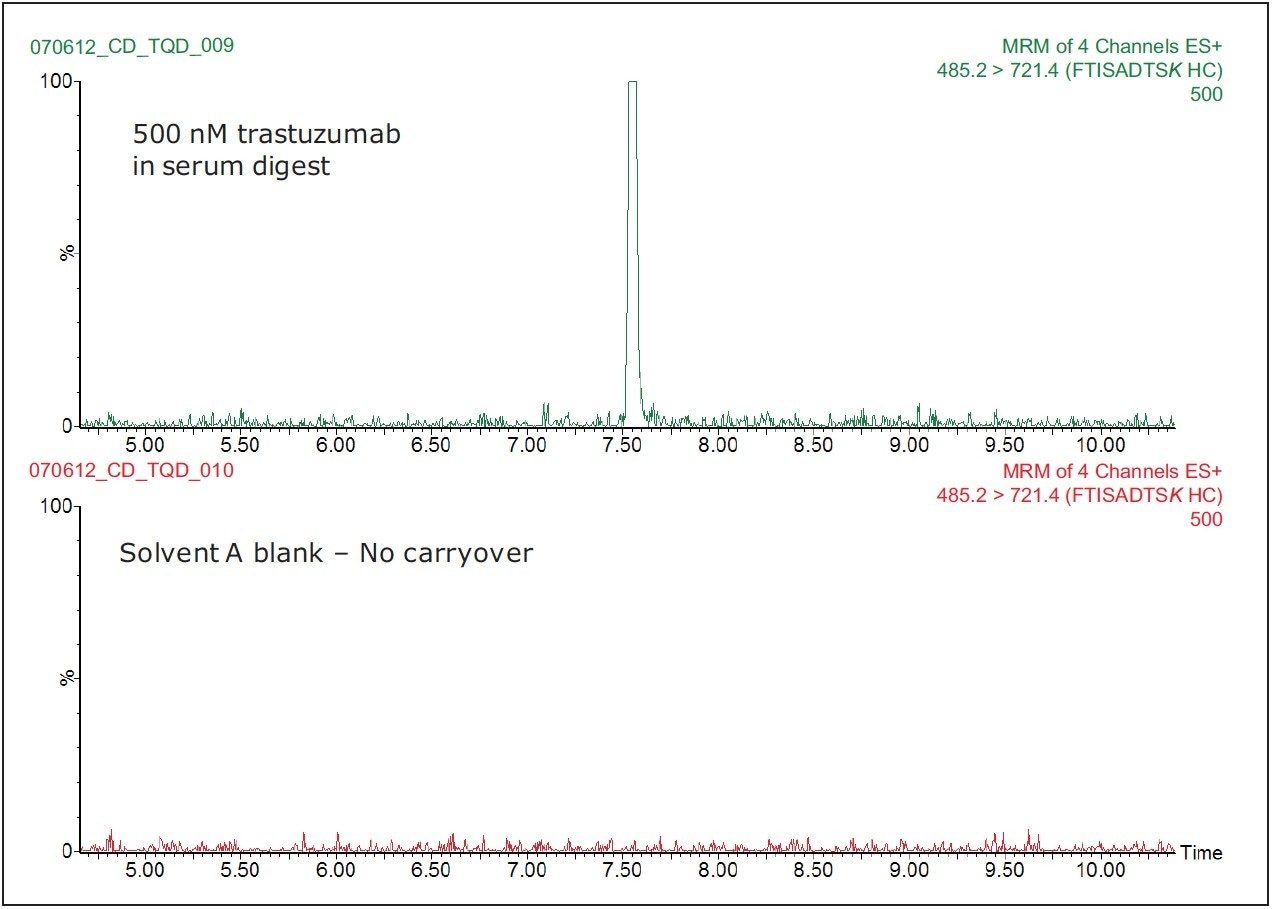
Figure 4 shows the SRM chromatograms of a Solvent A blank run, following the injection of the highest standard tested (500 nM trastuzumab in human serum digest). We can see from this data that there is no discernable carryover in the blank chromatogram (where the baseline has been magnified) for the FTISADTSK peptide. The extremely low carryover exhibited by the ACQUITY UPLC H-Class System allows the full sensitivity of the Xevo TQD Mass Spectrometer to be exploited.
720004423, July 2012