
This application note demonstrates accelerated, automated development of robust LC methods within a QbD framework.
The goal of this work is to demonstrate an accelerated method development approach using a Design of Experiments-based Quality by Design (QbD) methodology to develop HPLC and/or UPLC methods. Resulting methods are optimized for performance and robustness, ensuring success in final method validation and ultimately in method transfer.
The process of drug development produces samples of varying complexity with specific analytical requirements. The associated method development efforts that take place throughout a pharmaceutical organization can be a costly and time-consuming process. Streamlining the method development process can potentially allow these organizations to bring products to market faster and in a more cost-effective manner.
A myriad of approaches can be used to develop chromatographic methods, including manual trial and error (one factor at a time), software-based first principles, a simplex optimization, and design of experiments (DOE). Of these, only DOE can identify and quantify the complex interaction effects between method variables, in alignment with ICH Q8 (R2) Pharmaceutical Development.
A demonstrative method development example was carried out using a fullyautomated and integrated system consisting of Fusion AE Method Development Software, Empower 2 Chromatography Data Software (CDS), and an ACQUITY UPLC System with a photodiode array (PDA) detector, Column Manager, and Solvent Select Valve. This system configuration allowed for the screening of up to four different column chemistries, six different aqueous buffers/pHs, and two different organic mobile phases in one experiment (Figure 1).

Fusion AE is Quality by Design-based LC method development software with built-in robustness metrics. Fusion AE interfaces with the Empower 2 CDS, which controls the ACQUITY UPLC System. Using the chromatographic results from the Empower 2 CDS, Fusion AE manages complex statistics and automates method screening and 2 Analysis of Intact Lipids from Biologics Matrices by UPLC/Ion Mobility TOF-MS optimization. It builds experiments, analyzes data, and presents results as visual and numerical method predictions.
Fusion AE uses a logical workflow (Figure 2) that leads the user through the entire development process of designing the experiment and obtaining an optimized analytical method with a defined Design Space.
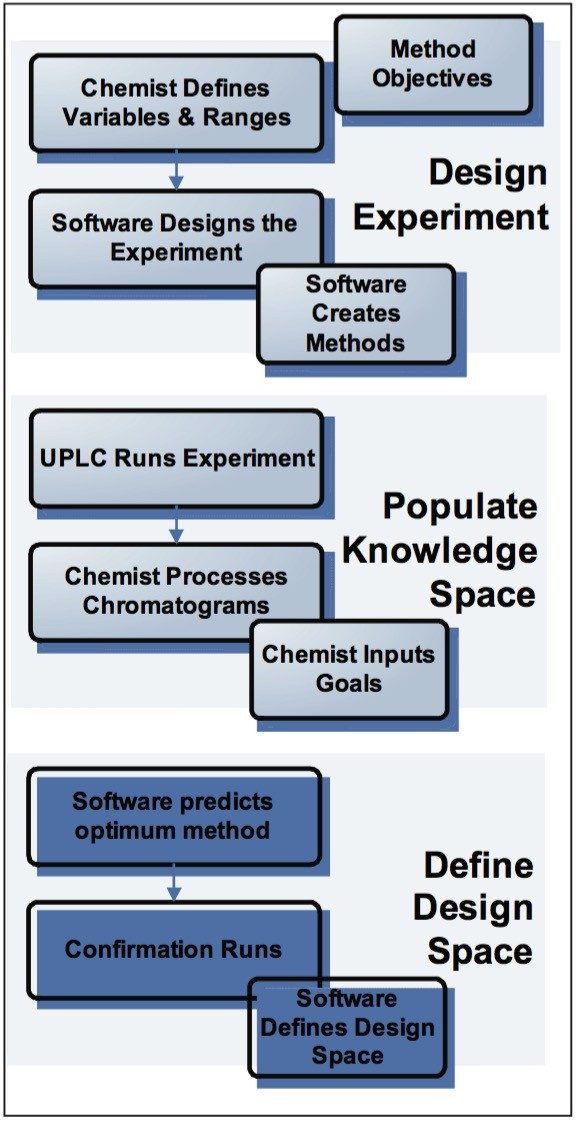
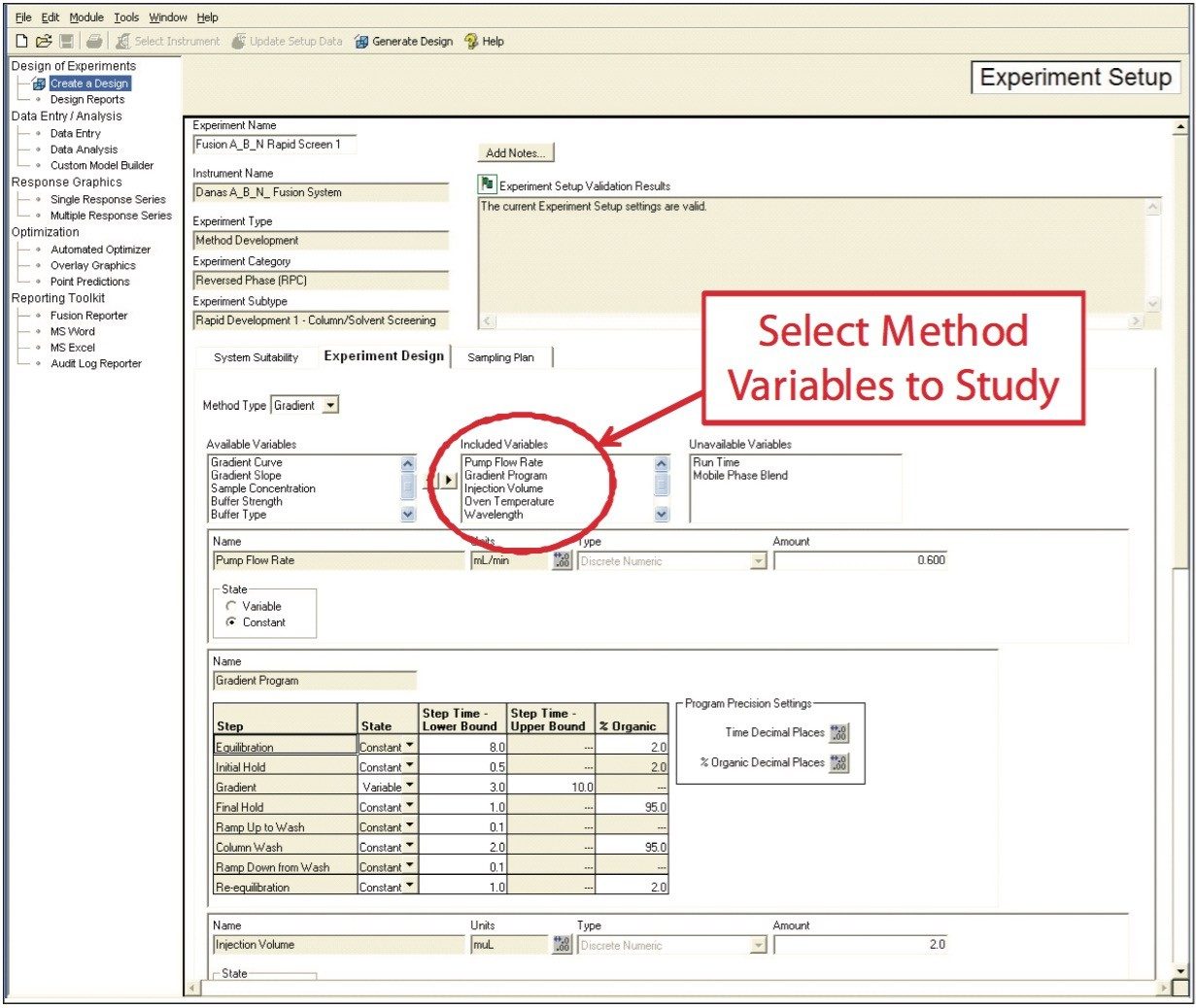
In the first step, Fusion AE automatically creates experiments that develop and optimize LC methods using standard or user-customized templates. Any combination of instrument parameters to study can be selected from the available variables list (Figure 3). The software constructs an Experimental Region and selects the most efficient statistical experimental design. Fusion AE then exports the experimental design to Empower 2 CDS, automatically creating all the instrument methods, method sets, and sample sets necessary to carry out the experiment and populate the knowledge space.
The ACQUITY UPLC System is used to run and process the collected chromatographic data, and the results are imported back into Fusion AE, which statistically analyzes and models the method performance responses into a quantitative Design Space. Data is quickly interpreted in reports and graphics for easy visualization of method results and interactions between variables.
Method development with Fusion AE is accomplished in two phases:
Fusion AE quantitatively evaluates method robustness without running additional experiments and identifies methods that are optimized for both mean performance and method robustness. Considering robustness during the method development phase, as recommended in the ICH Q2A guidance, can save considerable time and resources, and can give confidence that the method will pass validation and/or method transfer.
|
LC system: |
ACQUITY UPLC |
|
Columns: |
ACQUITY BEH C18, 2.1 x 50 mm, 1.7 μm ACQUITY BEH Shield RP18, 2.1 x 50 mm, 1.7 μm ACQUITY BEH Phenyl, 2.1 x 50 mm, 1.7 μm ACQUITY HSS C18 SB, 2.1 x 50 mm, 1.8 μm |
|
Buffers: |
10 mM Ammonium Formate, pH 3.0 10 mM Ammonium Acetate, pH 6.5 10 mM Ammonium Bicarbonate, pH 9.0 |
|
Organic mobile phases: |
Acetonitrile Methanol |
|
Gradient: |
2% B to 95% B |
|
Gradient time: |
3 min lower bound 10 min upper bound |
|
Flow rate: |
0.25 to 0.60 mL/min |
|
Column temp.: |
35 °C to 60 °C |
|
Gradient range: |
2% B to 80% B lower bound 2% B to 95% B upper bound |
|
Gradient time: |
2 min lower bound 6 min upper bound |
The next phase was to run a Method Optimization using the column and mobile phase selections determined from the Rapid Screen. An experimental design was created to optimize for the secondary effectors of selectivity:
|
Flow rate: |
0.25 to 0.60 mL/min |
|
Column temp.: |
35 °C to 60 °C |
|
Gradient range: |
2% B to 80% B lower bound 2% B to 95% B upper bound |
|
Gradient time: |
2 min lower bound 6 min upper bound |
In order to demonstrate this method development workflow, a mixture of 11 acidic, basic, and neutral compounds was prepared and a UPLC method was developed using Fusion AE. A rapid screening experiment was run evaluating four column chemistries, three buffer pHs, two organic mobile phases, and gradient time.
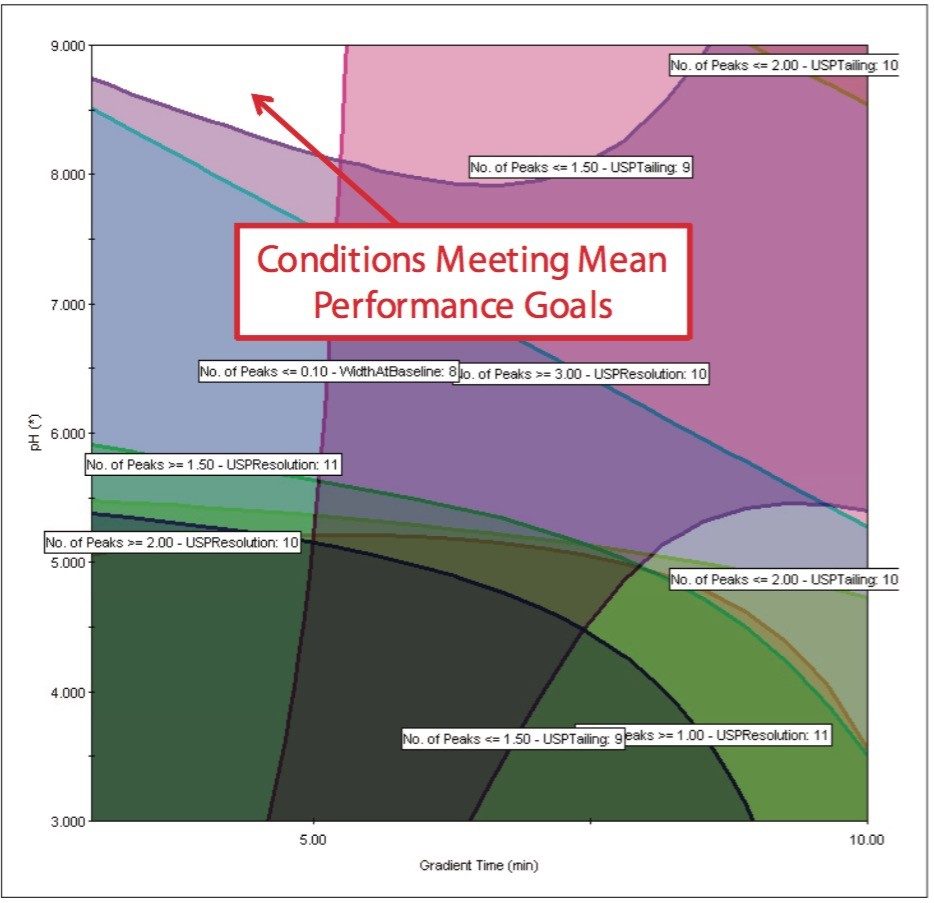
After running the experimental design on the ACQUITY UPLC System, the results were imported into Fusion AE and analyzed. The Automated Optimizer used the goals set for the method and determined the best conditions to be the ACQUITY UPLC BEH C18 Column with pH 9.0 buffer, acetonitrile as the organic mobile phase and a gradient time of 3 min (Figure 4). The results for the C18 column are easily visualized using the overlay graph (Figure 5). The unshaded region indicates the conditions where all of the mean performance goals were achieved.
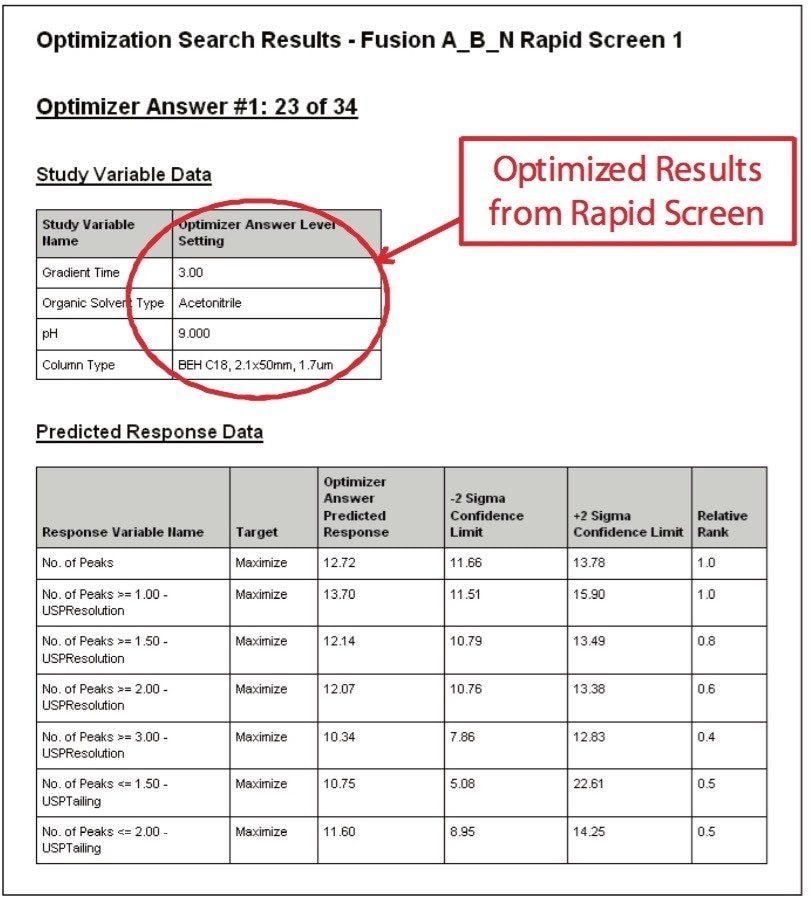
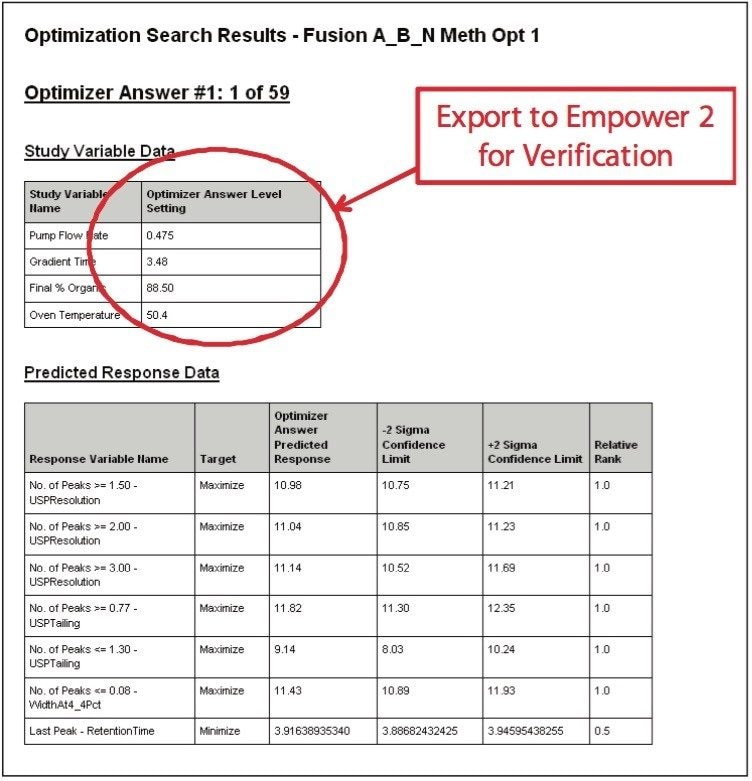
The UPLC results obtained for this optimization run were analyzed in Fusion AE. Different types of interactions between variables including linear additive effects, simple interactions, and complex interactions were observed using the Multiple Response Surface Plots and the Multiple Response Effects Plots (Figures 6 and 7). Goals for the method were set for number of peaks, USP resolution of peaks, peak widths, USP tailing, retention time of the last peak, along with robustness measurements for these responses. The Automated Optimizer calculated the best conditions to meet our mean performance goals and robustness criteria and identified the predicted results for these conditions (Figure 8).
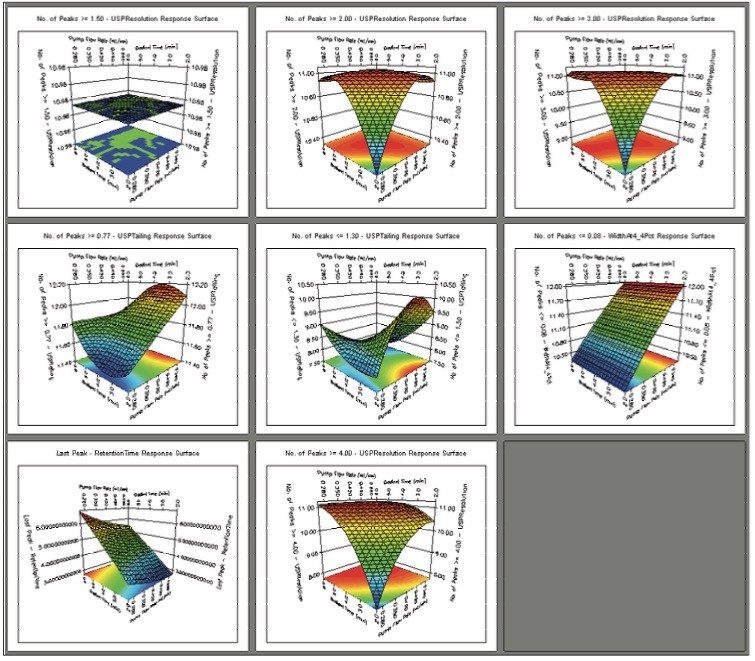
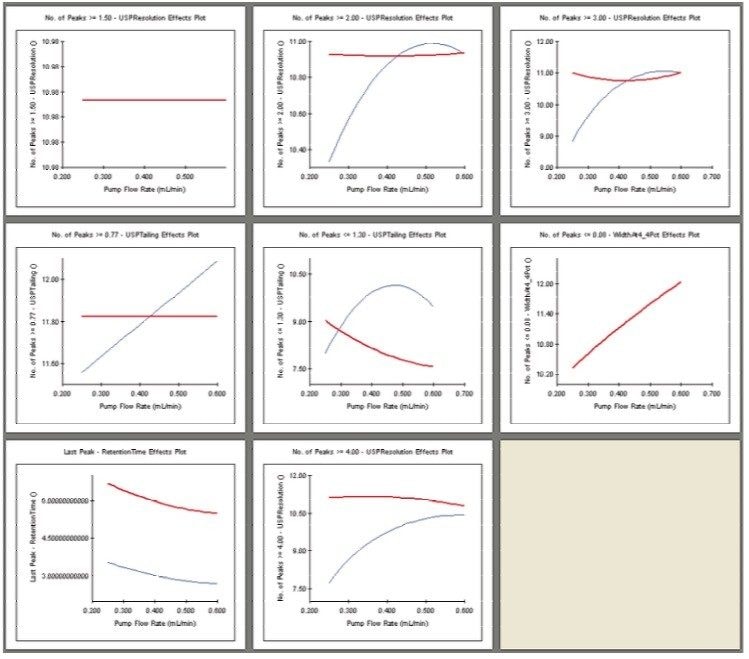
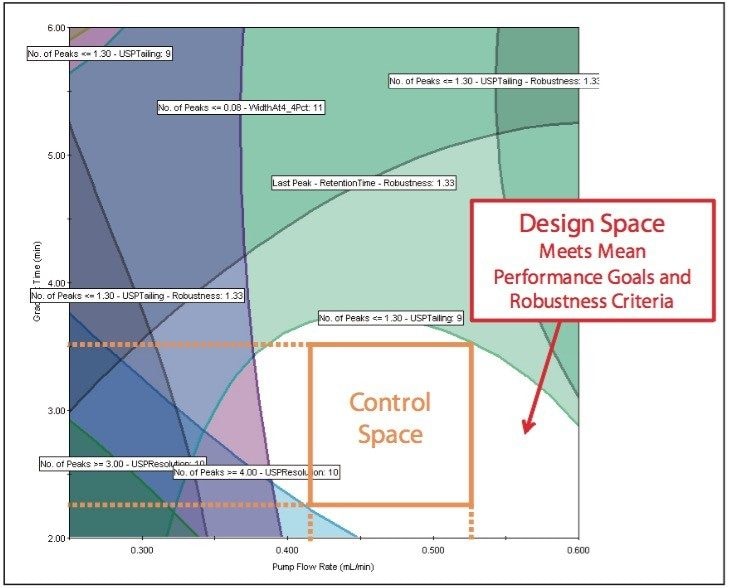
The Overlay Graph (Figure 9) clearly shows within the unshaded region the conditions where our mean performance goals and robustness criteria are achieved, defining the Design Space. Within the Design Space a square region can be selected to define the Operating Space where any change in the conditions within this region would not be considered a change in the chromatographic method based on our interpretation of ICH Q2A.
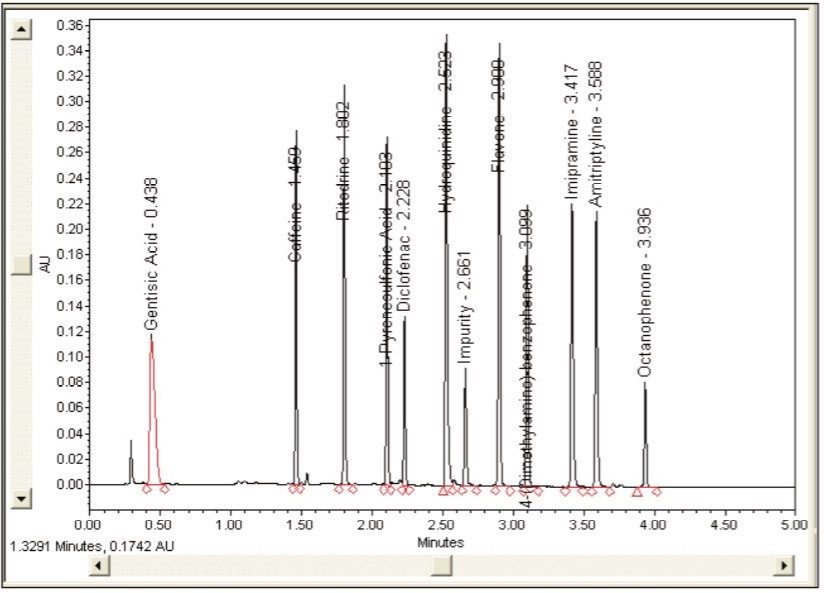
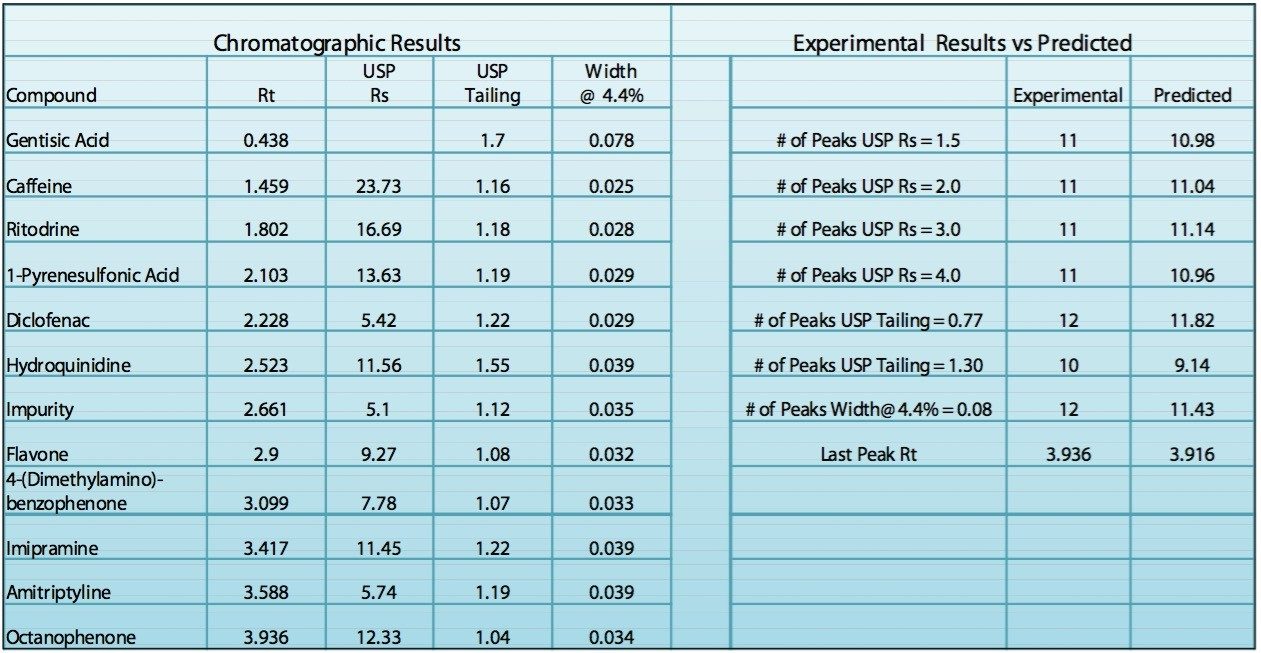
In order to verify that the optimized method will perform as expected, the Automated Optimizer prediction was exported to Empower 2 and run on the ACQUITY UPLC. The resulting chromatogram (Figure 10) shows an excellent separation in less than 5 minutes with good resolution between all 11 compounds (including an impurity) and good peak shape. Comparing the result table (Figure 11) with the predicted results from the Automated Optimizer indicates that the experimental results all meet or exceed the predicted results for the optimized method.
This entire method development process, including the Rapid Screening and the Method Optimization, required two days to obtain a final method.
Fusion AE Method Development Software with Empower 2 CDS and ACQUITY UPLC provides an ideal platform for method development using a QbD with Design of Experiments approach allowing scientists to develop the best possible methods faster and with greater confidence and method knowledge.
Using Fusion AE in combination with ACQUITY UPLC, the time required to develop optimized, robust LC methods can be reduced from weeks/months to days. The use of ACQUITY UPLC or ACQUITY UPLC H-Class systems dramatically increases the speed of the method development process while reducing solvent consumption for an overall increase in productivity and decrease in laboratory costs.
720003463, April 2010