
This is an Application Brief and does not contain a detailed Experimental section.
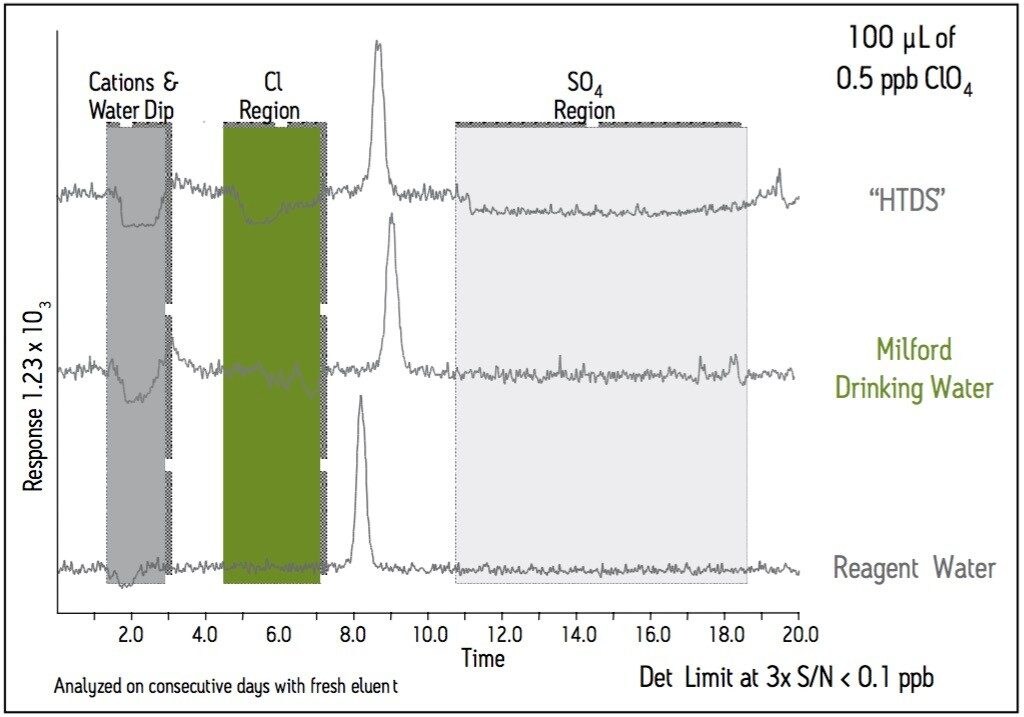
This application brief demonstrates the determination of perchlorate in water, soils and solid wastes using High Performance Liquid Chromatography/Electrospray Ionization/Mass Spectrometry (HPLC-ESI/MS).
Perchlorate is both naturally occurring and man-made. In its natural form, perchlorate is a contaminent in fertilizers. Man-made perchlorate is used in a wide variety of industrial applications including the production of rubber and paint, in lubricants, and as a primary ingredient in solid rocket propellant. Perchlorate is highly water soluble and can migrate into groundwater and surface water, posing a concern to drinking water supplies. Thirty-five states have detected perchlorate in drinking water at higher levels than expected. The United States Environmental Protection Agency (US EPA) has established an official reference dose of 0.0007 ppb per day of perchlorate. Maryland, Massachusetts and New Mexico have established a one part per billion (ppb) action limit, while California and Texas have established 4 ppb limits.
|
Instrument: |
Waters Alliance 2695 System with Conductivity Detector |
|
Column: |
IC-Pak Anion HR, 6 μm , 4.6 x 75 mm |
|
Eluent: |
25 mM NH4HCO3, pH 10 with NH4OH in 50% ACN |
|
Flow rate: |
0.5 mL/min |
|
Column temp: |
30 °C |
|
Back pressure: |
<1000 psi |
|
Back conditions: |
~1600 μS |
|
Injection: |
100 μL |
|
Instrument: |
Quattro micro Mass Spectrometer |
|
Ionization: |
ESI- LM 1 Resolution: 15 |
|
Capillary (V): |
.58 HM 1 Resolution: 15 |
|
Cone (V): |
40 Ion Energy 1: 0.6 |
|
Extractor (V): |
3 Entrance (V): 1 |
|
RF Lens (V): |
0.3 Collision Energy: 30 |
|
Source temp ˚C: |
125 Exit: 1 |
|
Desolvation temp: |
400 LM 2 Resolution: 14 |
|
Cone Gas (L/hr): |
50 HM 2 Resolution: 14 |
|
Desolvation gas: |
500 Ion Energy 2: 1 |
|
Gas cell pressure: |
2 x 10-2 mbar Multiplier: 650 |
720002735, August 2008