Efficient Profiling of Lipid Nanoparticle Formulations Using Waters GTxResolve 2000 Å SEC Column, MaxPeak Premier 3 µm
Abraham Samuel Finny, Lavelay Kizekai, Christian Reidy, Mandana Fasth, Balasubrahmanyam Addepalli, Matthew Lauber
Waters Corporation, United States
Published on May 05, 2025
Abstract
Lipid nanoparticles (LNPs) are used to deliver RNA therapeutics into cells. LNPs vary in size (a critical quality attribute, or CQA) and polydispersity depending on their nucleic acid cargo, lipid components, and manufacturing process. Careful control of their size and mass is critical to their biodistribution, overall efficacy and tissue targeting. This application note introduces the Waters™ GTxResolve™ 2000 Å SEC Column, MaxPeak™ Premier 3 µm as a tool for assessing the size profile of LNPs. A size exclusion chromatography (SEC) method has been achieved that provides profiling of two different LNP formulations. Fit-for-purpose SEC performance was facilitated by the high-performance surface hardware and carefully designed, pore-optimized packing material of the Waters GTxResolve 2000 Å SEC Column, which collectively provide excellent inertness, sizing capability and reproducibility with low salt mobile phase conditions. As such, this column can be used to quickly measure the size profile of LNP samples.
This application note demonstrates the feasibility of using a simple, low ionic strength mobile phase—0.1x Dulbecco’s Phosphate-Buffered Saline (DPBS)—for effective SEC-UV analysis of LNPs. The performance of Waters GTxResolve 2000 Å SEC Columns was highlighted under these conditions, showing that they can meet the demands of modern LNP analysis in terms of resolution and reproducibility. SEC analysis thereby provides drug developers with yet another option to comprehensively characterize LNPs. The data derived by SEC complements and corroborates the biophysical data that can be orthogonally gathered by dynamic light scattering (DLS), electrophoretic light scattering (ELS), and field-flow fractionation (FFF).
Benefits
- Superior resolution for large LNP species as afforded by Waters GTxResolve 2000 Å SEC Column particles and their optimal average pore diameter and finely tuned pore size distribution.
- Enhanced recovery under low ionic strength conditions as facilitated by novel column and packing material surfaces that are designed to be both hydrophilic and non-ionic.
- Fast, platform-compatible SEC methodology as made possible by 3 µm particle technology, high efficiency packed beds, and an overall column design that supports orthogonal high-sensitivity UV, MALS, dRI, and fluorescence detection for integrated LNP characterization.
Introduction
Due to their ability to encapsulate and protect fragile nucleic acid cargo, lipid nanoparticles (LNPs) have emerged as a transformative delivery system for mRNA, sgRNA, and siRNA.1 While the success of mRNA vaccines for COVID-19 underscores the critical role of LNPs in modern therapeutics, it remains essential to continue advancing the ways in which they can be characterized so that new breakthroughs in targeted delivery, stability, and potency can be uncovered.2
LNPs present a unique challenge to analytical scientists due to their diverse formulations, large hydrodynamic sizes, and complex stability profiles. With the increasing prominence of LNP-based therapeutics, particularly for mRNA and gene therapy applications, there is a growing need for reliable and readily accessible analytical techniques capable of resolving LNPs across a wide range of particle hydrodynamic radii while maintaining their sample integrity. Dynamic light scattering (DLS) and field-flow fraction (FFF) are commonly employed for LNP size analysis. DLS provides a rapid assessment of particle size, though it can lack the resolution to accurately quantify polydisperse samples like LNPs. Meanwhile, FFF delivers gentle, size-based separation, and high-resolution multi-attribute quantification of LNPs ranging from nanometer to micron radius in a single experiment, without the need for a porous stationary phase.
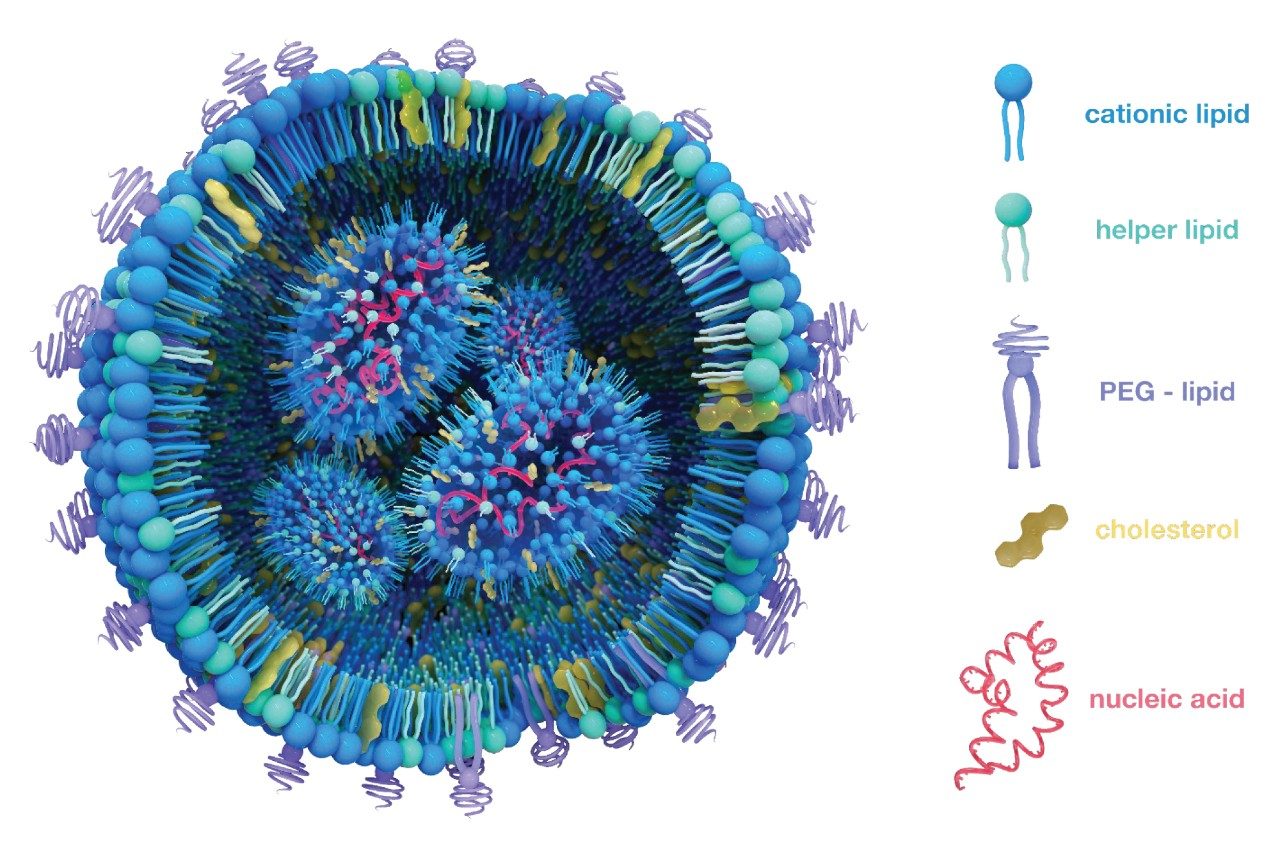
In contrast, SEC remains a preferred analytical platform for the development and release testing of biologics. It is regularly applied to the analysis of protein therapeutics and is becoming increasingly adopted for the analysis of the megadalton sized drug products in the cell and gene therapy industry. SEC is appealing because it is simple, reproducible, and compatible with UV, fluorescence, and multi angle light scattering (MALS) detection. That said, today’s SEC columns are being pushed to the limits of their capabilities when it comes to the analysis of large LNP species, which has necessitated advancements in both column technology, method considerations and mobile phase selection.3 In particular, low ionic mobile phases that mimic formulation diluent conditions are especially relevant for ex vivo quality control, release testing, and formulation stability assessment, etc. While mechanistic or biophysical studies that explore in vivo behavior may employ physiologically buffered conditions to simulate intracellular state, analytical methods intended to assess product quality and shelf-life stability benefit from using gentle, low-salt mobile phases that preserve native particle structure and avoid salt-induced aggregations. If optimal mobile phase conditions are applied, the particle integrity of the LNP can be preserved while maintaining compatibility with downstream analytical technique.4 This application note explores the performance of Waters GTxResolve 2000 Å SEC Columns for LNP analysis under low ionic strength conditions. Herein, the use of 0.1 x DPBS as a suitable mobile phase for size exclusion chromatography-based analysis while maintaining particle integrity was reported, achieving reproducible recoveries, and providing sizing profiles of LNPs. Further, these observations strongly indicate the possibility of using SEC as an orthogonal method to rapidly probe the LNP characteristics such as polydispersity, stability, and size distributions.
Experimental
A low ionic strength mobile phase was prepared by diluting sterile-filtered 1 × HyClone Dulbecco's Phosphate Buffered Saline (Ca²⁺/Mg²⁺-free, 0.1 µm filtered; Cytiva Catalog No. SH30028.03 or SH30028.02) in Milli-Q® water (0.22 µm-filtered) to a final 0.1× concentration. The resulting solution was mixed thoroughly, followed by sonication, and used as-is for all chromatographic analyses.
Low Ionic Strength Mobile Phase Considerations
The choice of mobile phase in SEC is crucial, as it can significantly influence the integrity and detection of LNPs. Employing low-ionic strength mobile phases, such as diluted Dulbecco's Phosphate-Buffered Saline (DPBS), helps in preserving the native state of LNPs during analyses. High ionic strength mobile phases, typically in the range of 100–150 mM or greater, can compromise lipid nanoparticles (LNPs) during in vitro analysis by screening surface charges, promoting aggregation and disrupting colloidal stability. Ionizable lipids within LNPs are susceptible to salt-mediated charge screening at near-neutral pH, and increased risk of particle fusion or structural degradation.5 In contrast, low ionic strength buffers—such as 0.1 × DPBS—preserve LNP integrity and thereby reduce sample–column interaction, and yield reproducible, size-based separations with accurate quantification. This makes them the preferred choice for analytical workflows involving LNPs and other colloidal delivery systems. Special care should be applied if it is of interest to use these methods for the simultaneous detection of unencapsulated nucleic acid.
Sample Information
In this study, Comirnaty™ (Pfizer-BioNTech) [NDC 0069-2362-01] and Spikevax™ (Moderna) [NDC 80777-102-04] COVID-19 vaccine formulations were employed as reference materials to evaluate column performance. These vaccines utilize LNPs to deliver mRNA encoding the SARS-CoV-2 spike protein, with each formulation exhibiting distinct characteristics. Comirnaty and Spikevax share a common structural approach, encapsulating mRNA within LNPs composed of ionizable lipids, phospholipids, cholesterol, and polyethylene glycol (PEG)-lipids. However, they have different ionizable lipids: Comirnaty employs the ionizable lipid ALC-0315, while Spikevax utilizes SM-102. These differences influence the physicochemical properties and stability profiles of the respective LNPs, and analytical comparisons have revealed that, despite targeting the same antigen, Comirnaty and Spikevax exhibit variations in particle size distributions and mRNA payloads.6,7,8
Both samples were diluted 1:1 using the mobile phase, followed by gentle vortexing to ensure homogeneity. The samples were then transferred to TruView pH Control LCMS Certified Clear Glass Vials (12 × 32 mm, Screw Neck; Waters, Catalog No. 186005663CV) and gently vortexed again to ensure the sample was at the bottom with no air bubbles before injection.
LC Conditions
Note on Flow Cell Selection: A 5 mm titanium flow cell (ACQUITY™ PDA, Waters SKU: 205000613) was selected to minimize metal–analyte interactions during SEC-UV analysis of LNPs in phosphate-containing mobile phase (0.1 × DPBS). Titanium offers superior inertness compared to stainless steel, which prevents adsorption of phosphate groups, ionizable lipids, and nucleic acids, and minimizing issues like peak tailing, baseline drift, and metal ion leaching. Unlike a standard, light-guided analytical flow cell, the Titanium flow cell does not contain any significant amounts of PTFE surface area. This ensures accurate recovery, preserves peak shape, and provides stable detector performance—critical for the reliable characterization of sensitive LNP formulations.
|
LC system configuration: |
ACQUITY™ Premier System with: – Binary Solvent Manager (BSM) – Flow-Through Needle Sample Manager (SM-FTN) – Column Manager-A (CM-A) |
|
Detector: |
ACQUITY Premier eLambda PDA with 5 mm Titanium Flow Cell |
|
Column(s): |
– Waters GTxResolve 2000 Å SEC Column, MaxPeak Premier 3 µm 4.6 × 150 mm (SKU: 186011346) – Agilent Bio SEC-5 2000 Å , 5 µm, 4.6 × 150 mm |
|
Mobile phase A: |
0.1× DPBS (HyClone Dulbecco's Phosphate Buffered Saline, no calcium and no magnesium, 0.1 µm filtered; diluted with Milli-Q® water) |
|
Seal wash: |
10% Methanol diluted with Milli-Q® water |
|
Column equilibration: |
30 minutes with Mobile Phase A @ 0.4 ml/min flow |
|
Vials: |
TruView™ pH Control LCMS Certified Clear Glass Vials, 12 × 32 mm, Screw Neck (Waters, SKU: 186005663CV) |
|
Column temperature: |
35 °C |
|
Sample temperature: |
5±3 °C |
|
Flow rate: |
0.1 mL/min |
|
Seal wash: |
10% HPLC-grade Methanol / 90% Milli-Q® water (v/v) |
|
Samples & injection volume: |
Comirnaty: 2 µL Spikevax: 4 µL |
|
Gradient: |
Isocratic |
|
System control & data acquisition: |
Empower™ 3.8.x (tested with 3.8.0.2) |
|
Detection wavelength: |
260 nm |
|
Sampling rate: |
20 points/sec |
Results and Discussion
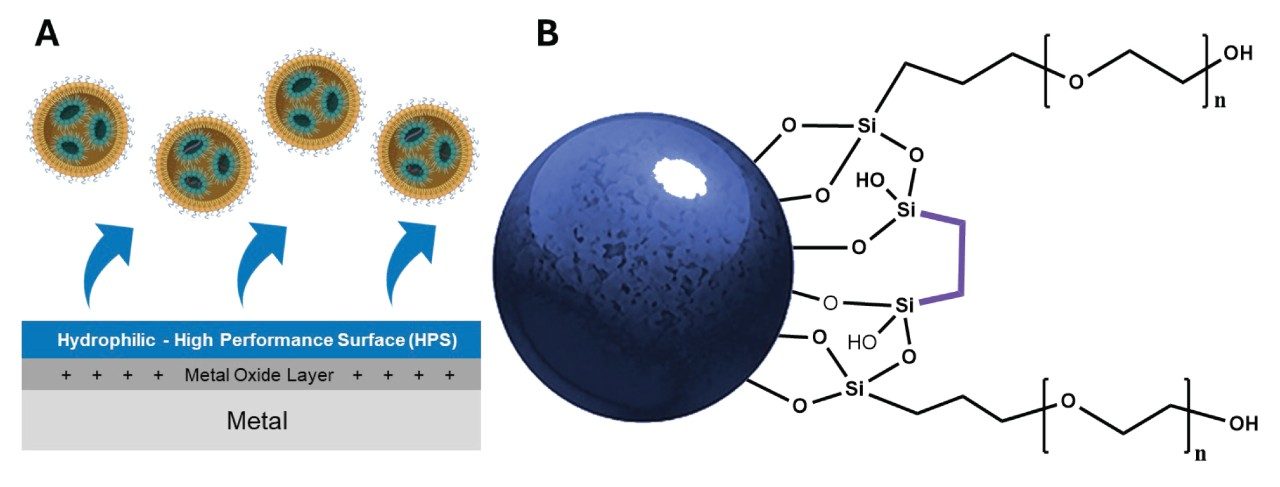
Waters GTxResolve 2000 Å SEC Column, MaxPeak Premier 3 µm Particles and Column Hardware Design
New column technologies were required to achieve reproducible profiling and characterization of large delivery vehicles such as LNPs. Figure 2 illustrates the key design features of the Waters GTxResolve 2000 Å SEC Column, optimized for the analysis of large biomolecules and nanoparticle-based therapeutics such as LNPs. One of the primary challenges in SEC analysis of these complex samples is the risk of analyte interaction with exposed metal surfaces in conventional stainless-steel hardware. Positively charged metal oxide layers can attract negatively charged sample components—such as certain ionizable lipids, nucleic acid payloads, and/or the net charge of an LNP—leading to non-specific adsorption, peak tailing, and inaccurate measurements. To overcome this, the Waters GTxResolve 2000 Å SEC Column incorporates MaxPeak High-Performance Surfaces (HPS). This proprietary surface treatment applies a hydrophilic, organic-inorganic hybrid barrier across all internal metal surfaces, dramatically reducing undesired analyte-surface interactions. By preventing metal-mediated adsorption, MaxPeak HPS enhances recovery, preserves peak shape, and ensures consistent performance, which is particularly critical under low ionic strength conditions like 0.1 × DPBS where electrostatic interactions are more pronounced. The use of 3 µm particles in SEC allows high-resolution LNP analysis in under 30 minutes using compact 4.6 × 150 mm columns. In contrast, columns packed with larger particles—such as 5, 8, 10, 26, or 27 µm—require longer columns and significantly longer run times, often exceeding 90 minutes for just a single injection.
In addition to advanced hardware, the Waters GTxResolve 2000 Å SEC Column features a novel 3 µm particle with an extra-large 2000 Å average pore size designed to resolve large, polydisperse analytes. The particle is comprised of high-strength silica, and the surface is chemically modified with a polyethylene oxide (PEO) bonding and bridged ethylene hybrid crosslinks, yielding a uniquely inert and hydrophilic packing material. This combination minimizes hydrophobic and ionic interactions while also improving chemical resilience and enabling high-efficiency separations. The MaxPeak HPS column hardware and wide-pore hydrophilic particle design make the Waters GTxResolve 2000 Å SEC Column a powerful tool for achieving highly reproducible, low-interaction separations of nanoparticle therapeutics, as shown in Figures 3 and 4.
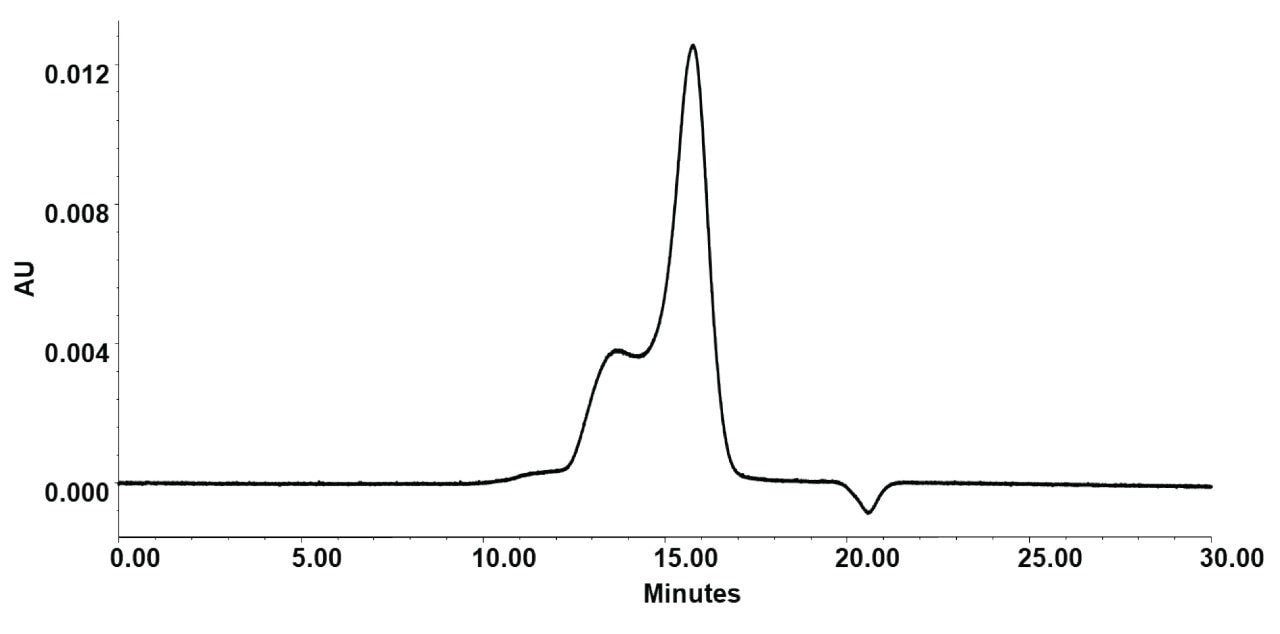

Reproducible LNP Separations and Recoveries
The LNP recovery in terms of total peak area obtained with the Waters GTxResolve SEC Columns was superior to conventional wide-pore SEC columns. The total peak areas of the LNP samples were significantly better (Figure 5) than those of commercially available steel hardware columns packed with similar pore-size particles. When benchmarked against such a column built with 5 µm, 2000 Å standard silica-based particles, the GTxResolve SEC Columns consistently showed higher total peak area recoveries. This superior, more reproducible recovery helps facilitate the analysis of even low-concentration LNP samples.
Waters GTxResolve 2000 Å SEC Columns delivered highly reproducible LNP separations over repeated injections across different batches and columns. Overlaying consecutive SEC-UV chromatograms of a given LNP sample showed virtually identical retention times and peak shapes for the main LNP peak, highlighting excellent run-to-run, batch-to-batch, and column-to-column consistency (Figure 5). In our evaluations, elution time variation among four different columns that were tested was negligible (%RSD=3.18), and peak area %RSDs remained low as well (5.84%). Such performance meets the requirements of a fit-for-purpose method. This level of reproducibility is critical for reliable characterization of LNP size distribution and purity, ensuring that any observed differences reflect actual formulation variations.
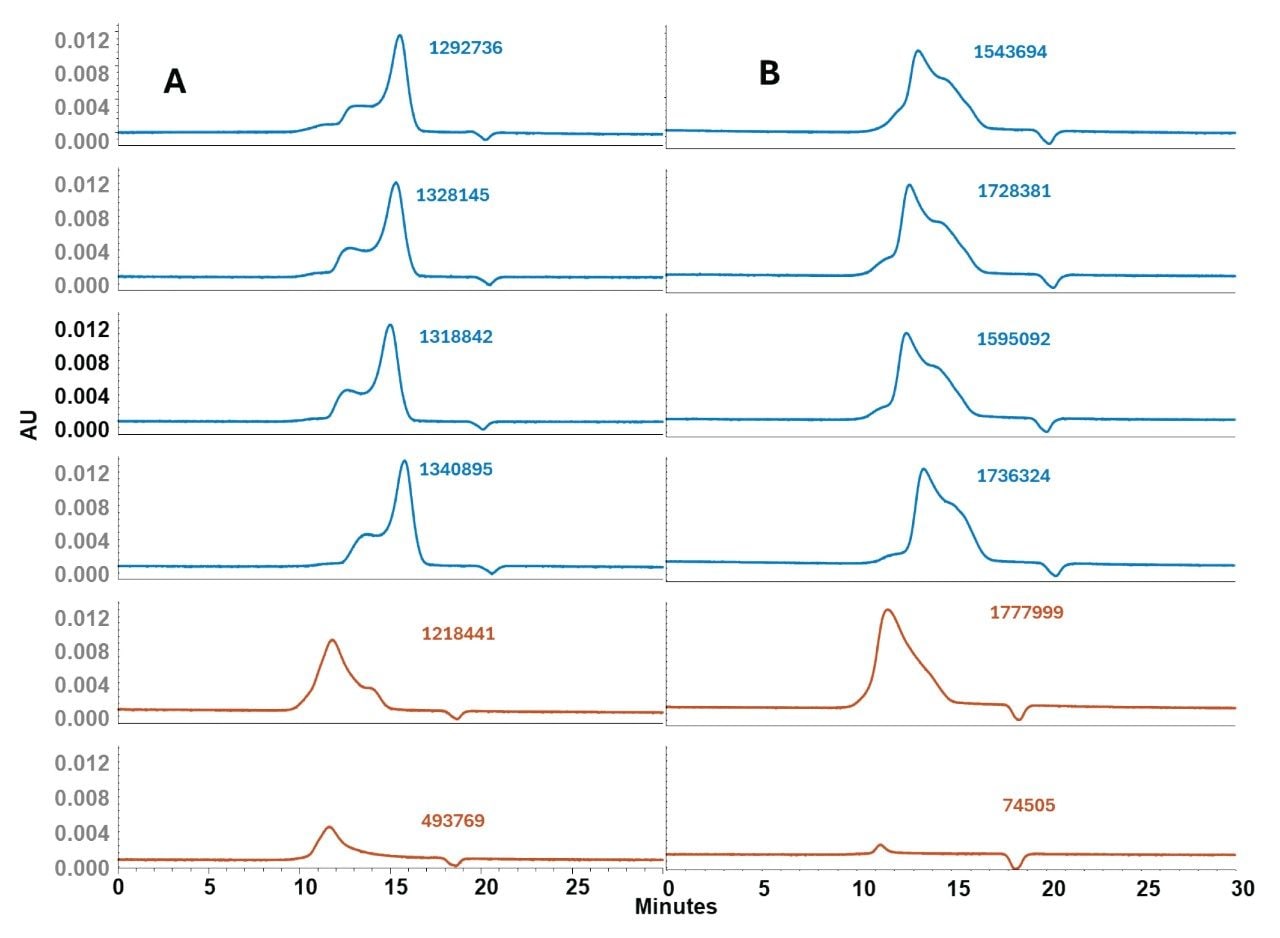
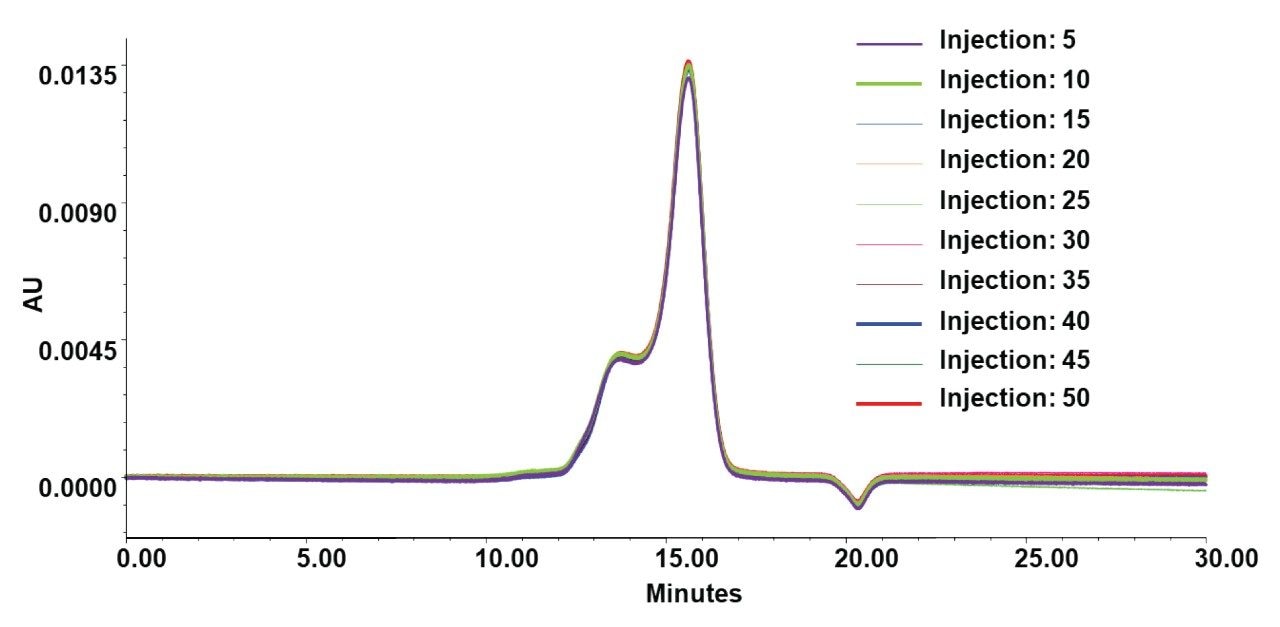
Column Lifetime
The stability of the GTxResolve SEC Columns as it relates to repeat injections of an LNP sample was also examined. A study was performed to monitor 50 consecutive LNP injections on Waters GTxResolve 2000 Å SEC Column. Figure 6 overlays these multiple injections. It can be seen that the LNP peaks align and that there was no broadening or emergence of spurious peaks. Key metrics such as elution time, total peak area, and the overall peak profile remained unchanged from the first injection through the 50th. The total peak area across the first 50 injections of LNP samples showed a %RSD of just 0.9%, demonstrating highly reproducible analyte recovery. This low variability indicates that the column surface exhibits minimal non-specific interactions with LNP components, even during early injections. Importantly, this level of consistency was achieved without any passivation, pre-conditioning, or use of non-ionic surfactants.
Through these experiences performing intact LNP SEC, it has been determined that the best practices can preserve long-term method performance. To further improve column lifetime and performance, it is helpful to perform a 10 µL injection of 6 M guanidine hydrochloride (GuHCl) after every five LNP injections to mitigate fouling. This cleaning step was incorporated as a best practice to preserve column performance and ensure consistent recovery.
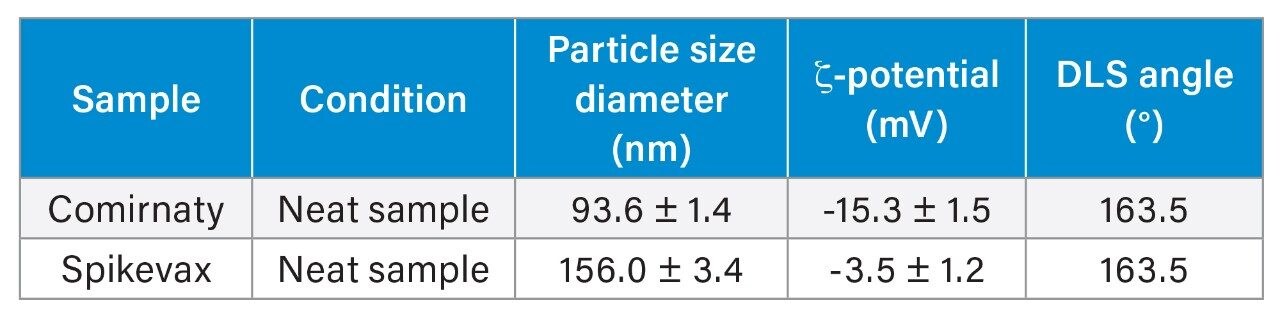
Comprehensive Analyses and Comparison with DLS
To ensure that the SEC-UV profiles corresponded to realistic and interpretable nanoparticle populations, the two LNP samples from this study were additionally characterized by dynamic light scattering (DLS). This orthogonal technique confirmed differences in particle size and zeta potential (an indicator of colloidal stability influenced by surface charge). Our sample of Comirnaty LNPs exhibited a smaller hydrodynamic diameter (94 nm) and a more negative zeta potential (–15 mV), while our sample of SpikeVax LNPs was found to be significantly larger (156 nm) with a near-neutral zeta potential (–3 mV). The size variations between the two formulations as highlighted by the DLS results correlate with the SEC profiles where earlier elution and broader peak shapes were observed for the larger SpikeVax LNPs. Importantly, despite their contrasting zeta potentials and inferred differences in surface chemistry, both Comirnaty and SpikeVax LNP formulations were successfully analyzed using the same SEC method (Figure 7).
In addition to DLS data, these SEC data can be compared and contrasted with previously published FFF measurements. A previous study on Comirnaty and Spikevax using FFF coupled with multi-angle light scattering (MALS), UV absorbance, and differential refractive index detection.9 The FFF results revealed significant differences in particle size distributions and mRNA payload content between the two LNP products, despite overlapping size ranges by MALS. Notably, Spikevax has a higher mass fraction of larger particles, as well as a greater average number of mRNA molecules per LNP. These findings also indicate that SEC-UV paired with an optimized wide-pore column provides a convenient screening tool for e.g., batch-to-batch variations and stability that offers higher resolution than batch DLS. SEC-UV offers relative quantification of peaks areas but cannot be used for comprehensive and absolute quantification of LNP quality attributes. SEC-UV screening can consequently be used as a complement to true high-resolution FFF-MALS-UV-RI multi-attribute quantification and mass spectrometry in e.g., a QC or development setting.
Considering Absolute Recovery
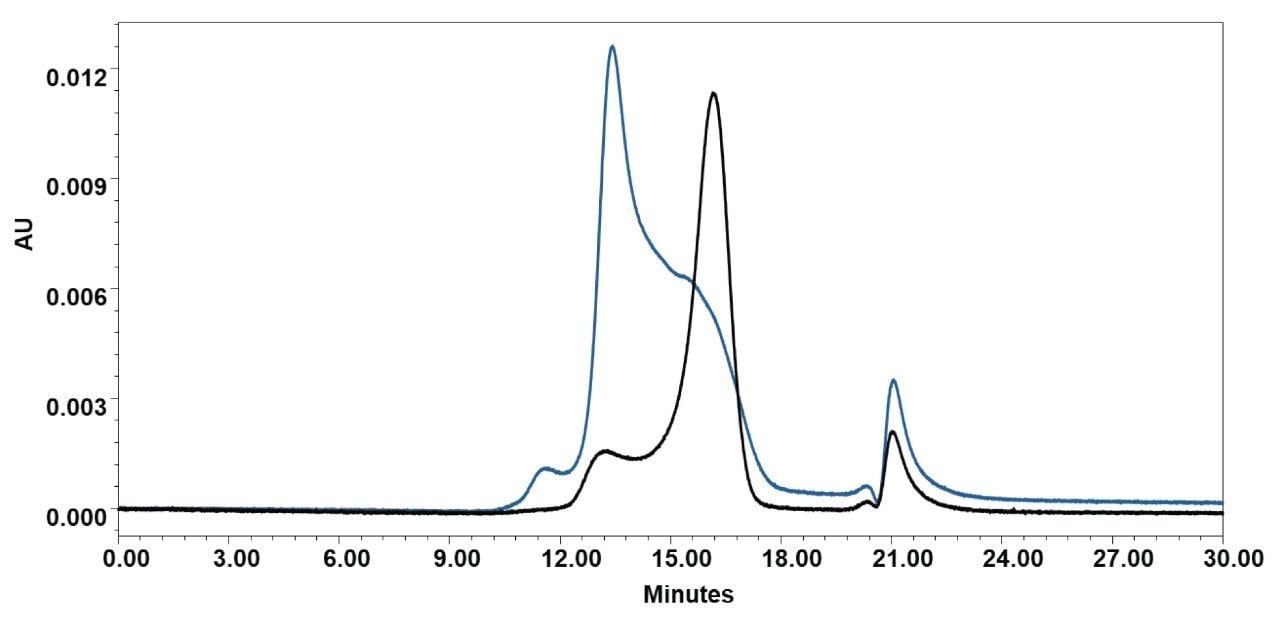
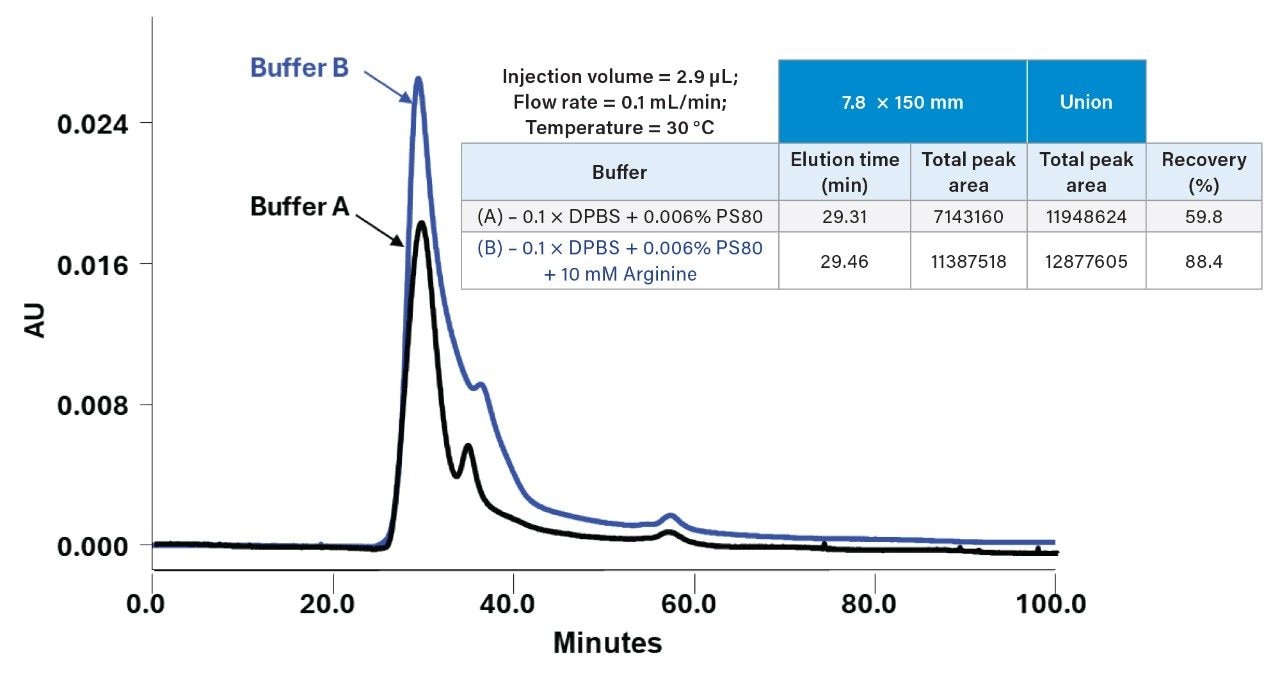
To evaluate absolute recovery, total peak areas (sum of all detected peaks) were compared between runs conducted with and without the Waters GTxResolve 2000 Å SEC Column, MaxPeak Premier 3 µm in line. This approach isolates column-specific losses, including analyte adsorption, retention, or structural disruption during separation. When analyzed using a 4.6 × 150 mm 2000 Å SEC Column operated at 0.1 mL/min with 0.1 × DPBS, the Pfizer Comirnaty LNP formulation showed a recovery of approximately 70.6%, whereas the Moderna Spikevax formulation recovered only 34.7%. These results suggest that while the Pfizer LNP is compatible with native SEC conditions, the larger and more surface-neutral Moderna LNPs may experience greater on-column interactions, aggregation, or loss—underscoring the need for formulation-specific method tuning. To explore potential improvements, mobile phase additives were tested using a 7.8 × 150 mm Waters GTxResolve 2000 Å SEC Column (Figure 8), while maintaining the flow rate at 0.1 mL/min.
Under these conditions, the addition of 0.006% polysorbate 80 (PS80) and 10 mM arginine to the mobile phase improved Spikevax recovery from 59.8% to 88.4%, illustrating the potential for excipients to mitigate any interactions. While the improved recovery may be attributed to the additive formulation, it is also possible that the lower linear velocity in the unscaled 7.8 mm format contributed to enhanced performance, suggesting that flow rate optimization may be required for improved recoveries. Nonetheless, any additive-based strategy must be applied judiciously. Surfactants or charge-masking agents can affect LNP structural integrity, and size distribution. Therefore, selection of mobile phase conditions should be guided by the analytical objective, where the goals is high-throughput recovery, product release testing, or quick profiling of the LNP species while preserving native particle properties for mechanistic evaluation. Ongoing efforts are focused on understanding any relationships between high recovery and structural fidelity, particularly for more challenging or heterogeneous LNP formulations. It will be interesting to compare these recovery measurements through orthogonal techniques such as FFF-MALS.
Conclusion
As part of a growing toolbox for the analysis of LNP drug substances, the use of Waters GTxResolve 2000 Å SEC Columns is proposed. Dynamic light scattering, electrophoretic light scattering, and field-flow fractionation can be relied upon as tried and true approaches for rapid screening and multi-attribute quantification, respectively. For orthogonal capabilities and screening studies, GTxResolve SEC Columns can now be considered too. These columns are uniquely engineered to meet the growing demands of LNP characterization, offering superior peak profiles and reproducibility for multiple types of formulations compared to other commercially available options. Its wide pore packing material allows for efficient separation of intact LNPs, while the combination of an inert polyethylene oxide surface chemistry and MaxPeak HPS hardware ensures minimal analyte loss and secondary interactions, even under low ionic strength conditions like 0.1 × DPBS, which has proven to be a viable mobile phase for repeatable LNP studies. Progress on SEC methods for LNPs is promising. While this preliminary study provides an intriguing perspective on things to come, it is future investigations that are bound to show a rich interplay between various analytical approaches and how to apply them to guide development teams toward more potent, more stable lipid nanoparticles.
As biopharmaceutical pipelines continue to evolve toward larger and more sophisticated delivery platforms, the Waters GTxResolve 2000 Å SEC Column, MaxPeak Premier 3 µm offers a fit-for-purpose SEC solution that balances analytical rigor with practical usability, making it a preferred tool for modern large-molecule separations.
References
- P. R. Cullis and M. J. Hope. “Lipid Nanoparticle Systems for Enabling Gene Therapies,” Mol. Ther., vol. 25, no. 7, pp. 1467–1475, Jul. 2017, doi: 10.1016/j.ymthe.2017.03.013.
- L. Schoenmaker et al., “mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability,” Int. J. Pharm., vol. 601, p. 120586, May 2021, doi: 10.1016/J.IJPHARM.2021.120586.
- V. D’Atri et al., “Size exclusion chromatography of biopharmaceutical products: From current practices for proteins to emerging trends for viral vectors, nucleic acids and lipid nanoparticles,” J. Chromatogr. A, vol. 1722, p. 464862, May 2024, doi: 10.1016/J.CHROMA.2024.464862.
- A. M. Börjesdotter et al., “Lipid nanoparticle properties explored using online asymmetric flow field-flow fractionation coupled with small angle X-ray scattering: Beyond average characterisation,” Int. J. Pharm., vol. 668, p. 124940, Jan. 2025, doi: 10.1016/j.ijpharm.2024.124940.
- S. Tang et al., “Influence of salt solution on the physicochemical properties and in vitro/ in vivo expression of mRNA/LNP,” J. Nanobiotechnology, vol. 23, no. 1, pp. 1–18, Dec. 2025, doi: 10.1186/s12951-025-03318-w.
- L. Zhang et al., “Effect of mRNA-LNP components of two globally-marketed COVID-19 vaccines on efficacy and stability,” npj Vaccines, vol. 8, no. 1, pp. 1–14, Oct. 2023, doi: 10.1038/s41541-023-00751-6.
- J. Szebeni et al., “Insights into the Structure of Comirnaty Covid-19 Vaccine: A Theory on Soft, Partially Bilayer-Covered Nanoparticles with Hydrogen Bond-Stabilized mRNA-Lipid Complexes,” ACS Nano, vol. 17, no. 14, pp. 13147–13157, Jul. 2023, doi: 10.1021/acsnano.2c11904.
- C. Hald Albertsen, J. A. Kulkarni, D. Witzigmann, M. Lind, K. Petersson, and J. B. Simonsen. “The role of lipid components in lipid nanoparticles for vaccines and gene therapy,” Advanced Drug Delivery Reviews, vol. 188. Elsevier, p. 114416, Sep. 01, 2022. doi: 10.1016/j.addr.2022.114416.
- M. Kurnik. “Multi-attribute quantification of LNP-mRNA therapeutics by FFF-MALS and DLS.” Accessed: Apr. 07, 2025. [Online]. Available: https://www.wyatt.com/library/application-notes/an2615-multi-attribute-quantification-of-lnp-mrna-therapeutics-by-fff-mals-and-dls.html.
720008797, May 2025