High-Throughput Reactive Metabolite Screening for Diclofenac by UPLC and Xevo TQ MS With ScanWave
Introduction
The study of reactive metabolites has been gaining more attention in modern drug development due to their instability (short-lived electrophiles) and high potential for drug-related toxicity. A common way to study reactive metabolites is to trap them with a nucleophilic reagent such as Glutathione (GSH). With the detection of the GSH conjugates, potential reactive metabolites can be identified.
There are a few MS experiments that are commonly used to screen for GSH conjugates.1–3 For positive electrospray ionization (ESI) experiments, a common neutral loss of m/z 129 (pyroglutamic acid moiety), 307 (aliphatic and benzylic thioethers), or 147 (glutamic acid for thioesters) can be monitored. For negative ESI experiments, the precursor ion of m/z 272 (g-glutamyldehydroanalyl-glycine) can be monitored to identify the GSH conjugates (Figure 1).
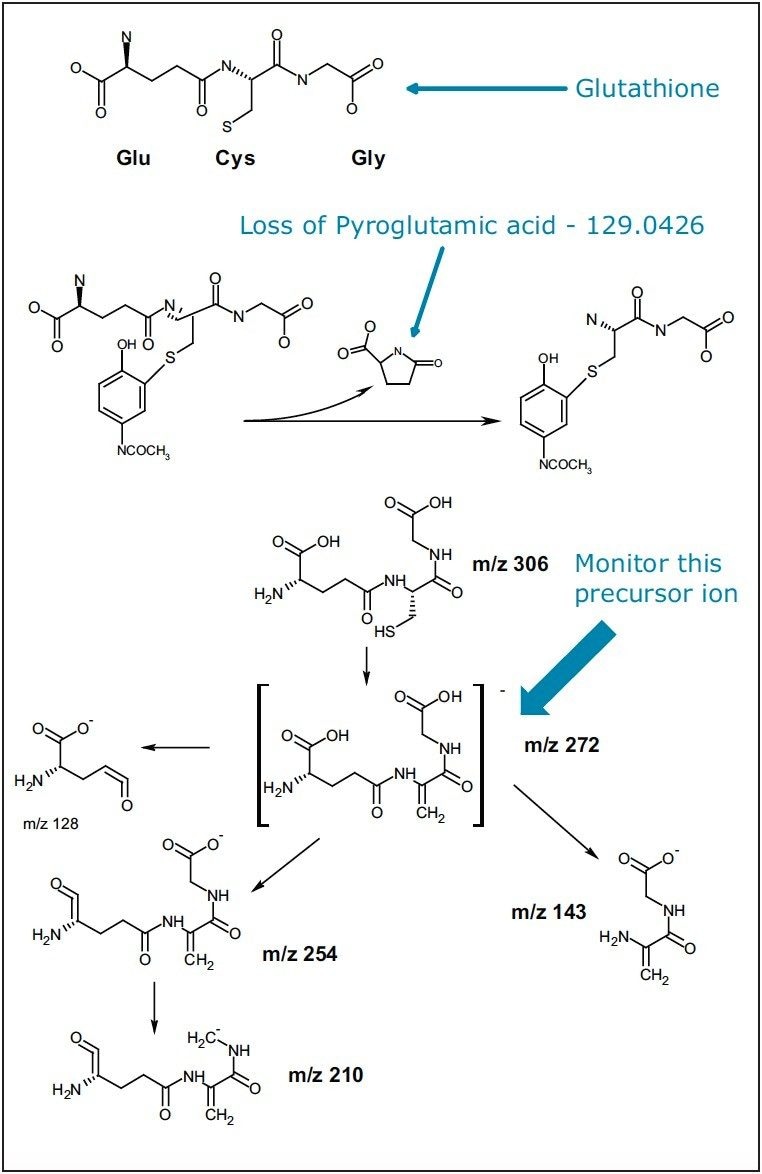
Here we present an approach for screening GSH conjugates using LC/MS/MS, where data from multiple MS/MS experiments can be acquired simultaneously by a tandem quadrupole MS in a single UPLC® run. This screening strategy is illustrated in Figure 2.
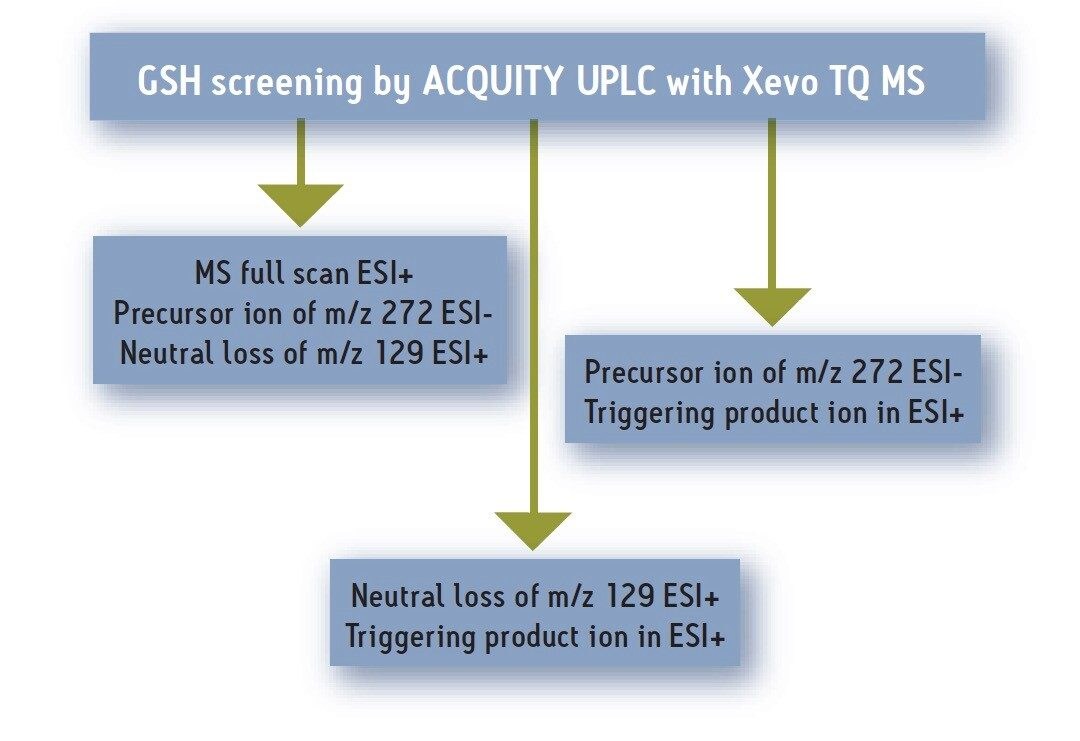
Diclofenac (DF) was used as the test compound. It was incubated with human liver microsome and GSH at 10 mm for 60 minutes. The sample was analyzed using a Waters ACQUITY UPLC® System with the Xevo™ TQ MS System equipped with ScanWave™ Technology. Examples from positive MS full scan, negative precursor ion scan, positive product ion scan, and the ability to automatically switch among these acquisition modes, along with the signal enhancement by ScanWave Technology, will be shown.
Experimental
Incubation with pooled human liver microsomes (2 mg/mL protein) was conducted in 50 mM potassium phosphate buffer (pH 7.4), at 37 °C in a incubator for 60 min. Diclofenac and 2 mM GSH were added into the buffer with a final substrate concentration of 10 µM. The incubation was initiated by NADPH generating system consisting of 0.44 mM NADP+, 5.53 mM glucose-6-phosphate and 1.2 units/mL glucose-6-phosphate dehydrogenase together with 3 mM MgCl2. The incubation was terminated at 60 min by the addition of 0.7 mL of ACN (with 6% of formic acid, with a total of volume of 1.7 mL). The quenched sample was then centrifuged at 2000 RPM for 20 min. 0.8 mL of the supernatant was dried under vacuum and then reconstituted into 0.08 mL of 30% ACN in water. 10 µL were injected in the ACQUITY UPLC/Xevo TQ MS system.
LC/MS Methodology
|
LC system: |
Waters ACQUITY UPLC System |
|
Column: |
ACQUITY UPLC HSS T3 C18 Column 2.1 x 100 mm, 1.8 µm |
|
Column temperature: |
65 °C |
|
Mobile phase A: |
0.1% Formic acid |
|
Mobile phase B: |
Acetonitrile |
|
Flow rate: |
0.6 mL/min |
|
Gradient: |
98% A to 40% A in 5 min, ramp from 40 to 0 %A in 1 min, stay at 0% for 2 min before returning to 98% A for re-equilibration |
|
Injection volume: |
10 µL |
|
MS system: |
Xevo TQ MS |
|
Acquisition range: |
50 to 700 Da |
|
Ionization mode: |
Positive and negative ion modes |
ScanWave Technology
Based on a novel use of Waters' proven T-Wave™ collision cell technology, Xevo TQ MS's ScanWave mode of operation enhances both MS and product ion spectral data. ScanWave operation is based upon two concepts (Figure 3):
- First, the front and back of the collision cell are independently controlled, which allows fragmentation and accumulation of ions to occur in the front of the gas cell, while previously accumulated ions are simultaneously ejected from the back of the gas cell. This provides 100 percent sampling efficiency
- Second, ScanWave links the low-resolution ion ejection from the gas cell with scanning of the final-resolving quadrupole (MS2). This enables an intelligent ion delivery where ions are presented to the final quadrupole when they are actually needed, rather than continuously as in traditional tandem quadrupole instruments
This novel ion delivery technique provides significant duty cycle improvements that, in turn, result in enhanced signal in scanning acquisition modes.

Results and Discussion
The major GSH adducts of diclofenac from the incubation with human liver microsomes were reported as the hydroxy (OH) glutathione-DF with a molecular weight of 616, and the hydroxy (OH) glutathione-deschloro-DF with a molecular weight of 582.4
Figure 4 shows the positive MS full-scan experiment, with a neutral of loss of m/z 129 and negative precursor ion of m/z 272 in the same LC/MS/MS run. The bottom trace (in green) is the MS full-scan chromatogram showing multiple peaks eluted between 1 and 7 minutes. Both the positive neutral loss scan and negative precursor ion scan chromatograms (in red) indicate that the DF GSH conjugate of 582 eluted at 2.80 minutes and 616 eluted at 2.93 minutes in this experiment.
An illustration of the instrument hardware for the corresponding scan modes is shown on the left side of the Figure 4. The ability to conduct multiple-function scanning experiments between positive and negative ion modes in a single LC/MS/MS run is very desirable because it enables the instrument to monitor GSH conjugates more efficiently and eliminates the detection of possible false-positive GSH conjugates obtained using a single-scan mode.
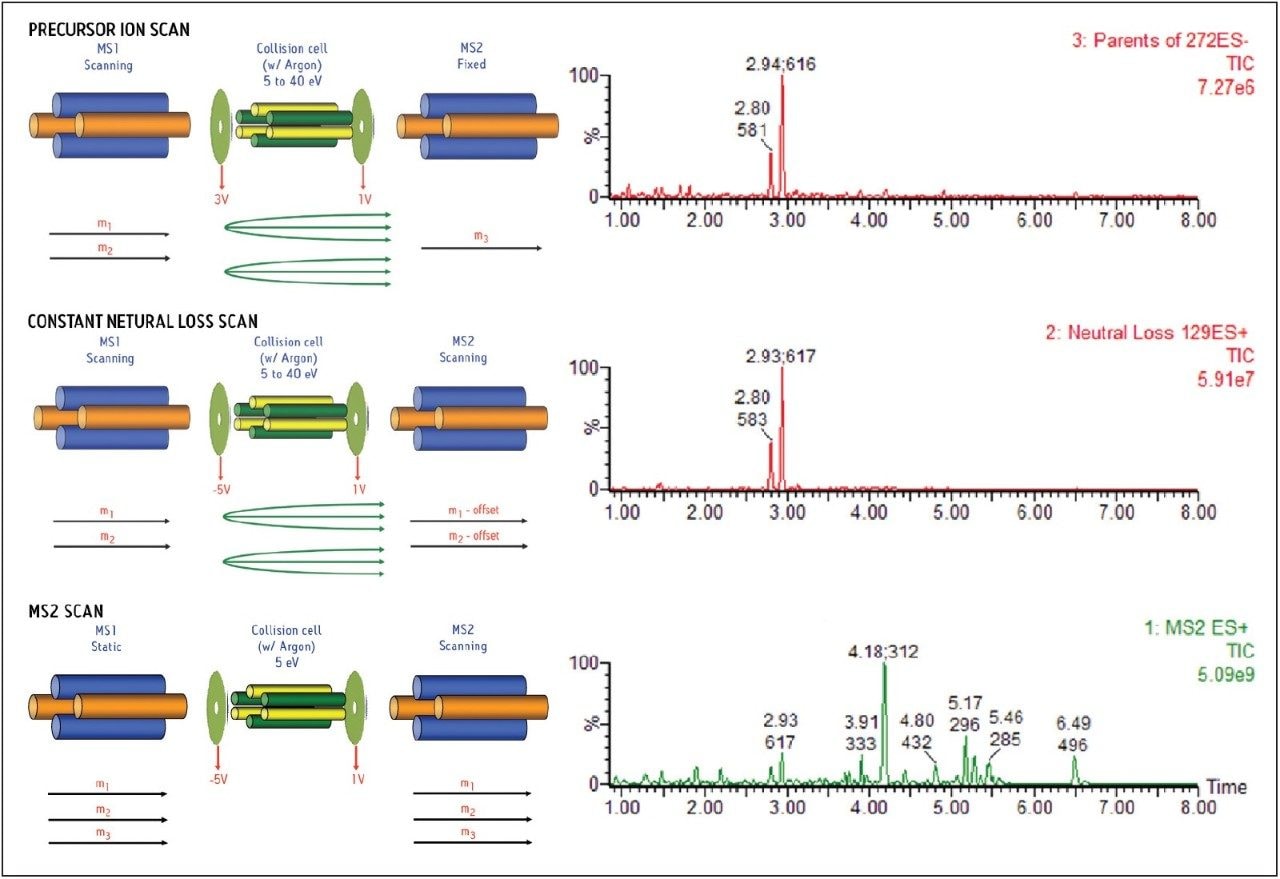
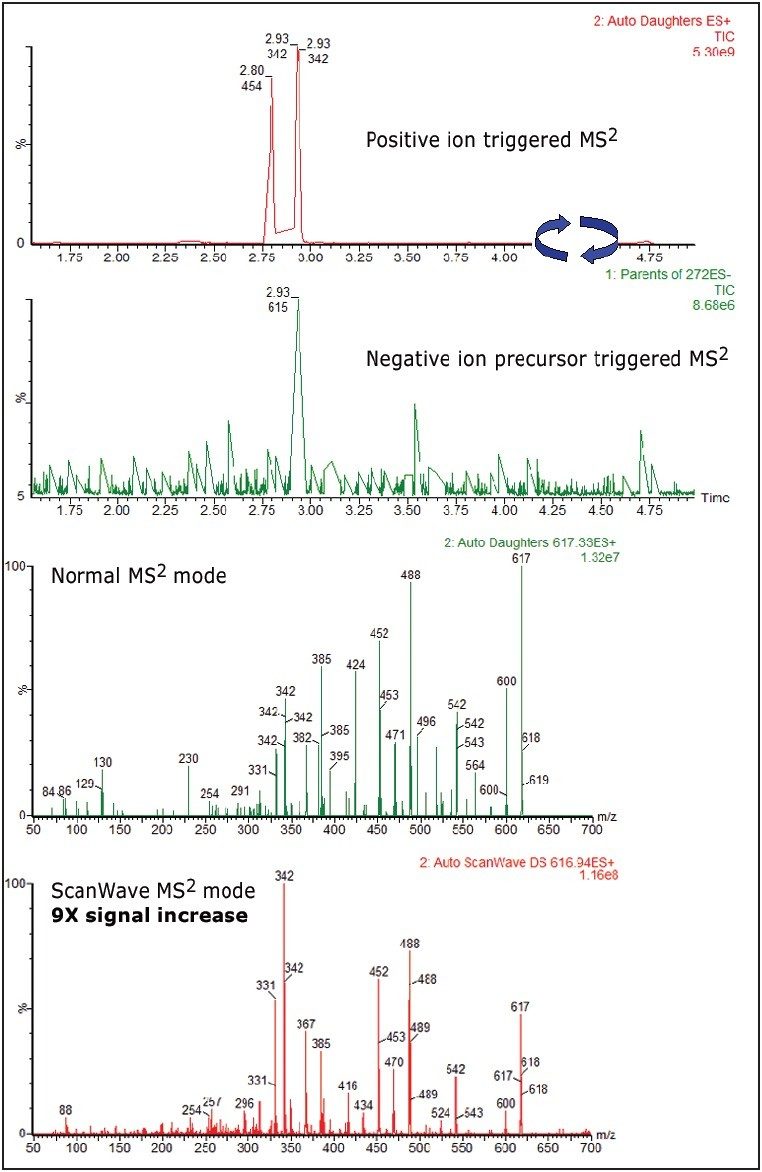
Figure 5 shows diclofenac GSH conjugate m/z 583 and 617 comparison spectra between normal MS2 scan mode and ScanWave MS2 scan mode, triggered from a positive-ion neutral loss of 129 survey scan. A six- to nine-fold increase in sensitivity was observed from the ScanWave MS2 scan mode experiments.

Figure 6 shows diclofenac GSH conjugate 583 and 617 comparison spectra between normal MS2 scan mode and ScanWave MS2 scan mode, triggered from a negative precursor ion of 272 survey scan mode. Again a six- to nine-fold increase in sensitivity has been observed from the ScanWave MS2 scan.
Conclusion
Using diclofenac as the test compound, the combination of an ACQUITY UPLC System with the Xevo TQ MS has been shown to be very effective in high-throughput reactive metabolite screening. The versatility of conducting multiple-function switching and survey scans between positive and negative ion modes in a single LC/MS/MS run enables more efficient monitoring of GSH conjugates, and eliminates the possibility of obtaining false positives from a single scan mode.
While this system provides the flexibility to carry out any of the strategies shown in Figure 2, it was found that negative ion precursor of m/z 272 with subsequent triggering to MS2 mode is the technique that may provide the widest coverage regardless of the location of the GSH adduct.5 The sensitivity increase from the ScanWave Technology provides great benefits in detecting low-abundance metabolites. The UPLC/Xevo TQ MS system delivers important contributions in quantitative as well as qualitative analysis in the drug discovery and development processes.
References
- Castro-Perez J. Applications of Quadrupole-Time-of-Flight Mass Spectrometer in Reactive Metabolite Screening. Mass Spectrometry in Drug Metabolism and Pharmacokinetics. 1st Edition. John Wiley and Sons, Inc. Hoboken, NJ, USA. 159–190. 2008.
- Zheng J, Ma L, Xin B, Olah T, Humphreys G., and Zhu M. Chem. Res. Toxicol. 20: 757–766. 2007.
- Ma S, and Subramanian R. Journal of Mass Spectrometry 41.10. 1121–39. 2006.
- Yu L, Chen Y, DeNinno M, O'Connell T, Hop C. Drug Metabolism and Disposition. 33[4], 484–488. 2005.
- Wen B, Ma L, Nelson S, Zhu M. Anal. Chem. 80, 1788–1799. 2008.
720003024, May 2009