UHPLC-MS/MS Analysis of Amino Acids in Dried Blood Spots using Waters Kairos Amino Acid Kit for Clinical Research
Rachel McBrinn, Gareth Hammond, Padhraic Rossiter, Lisa J. Calton
Waters Corporation, United States
Published on June 05, 2025
Abstract
This study details the quantification of 42 amino acids in dried blood spot (DBS) matrices using the Kairos™ Amino Acid Kit and AccQ•Tag™ Ultra “3X” Derivatizing Reagent. Analysis was performed with an ACQUITY™ UPLC™ I-Class PLUS System and a Xevo™ TQ-S micro Triple Quadrupole Mass Spectrometer. Method performance was evaluated using Kairos Calibrators and Quality Controls, external Centers for Disease Control and Prevention (CDC) DBS materials, and in-house Calibrator and Quality Control DBS materials.
The AccQ•Tag Ultra “3X” Reagent enabled accurate and precise quantification of amino acids in DBS samples, with a run time of 9 minutes per sample. This method provides clinical researchers with a simple and rapid analysis technique, eliminating the need for mobile-phase buffers or ion-pair reagents.
Benefits
- Analytical chromatographic method providing separation of isobaric species in <10 minutes
- 350 µL plate format
- Buffers and ion-pairing agents are not required
Introduction
Quantifying amino acids from DBS using optical detection typically requires complex mixtures of buffers and/or ion-pair reagents, along with lengthy analytical run times. This study demonstrates the utility of the RUO Kairos Amino Acid Kit for analyzing 42 amino acids in DBS using LC-MS-based technologies.
For comprehensive instructions on using the Kairos Amino Acid Kit (p/n: 176004379), refer to the Care and Use Manual (p/n: 720006448), which details sample preparation, analytical methodology, and performance characteristics for solvent Calibrators and Quality Controls. This study summarizes the method performance of Waters in-house DBS Quality Controls prepared with the Kairos Amino Acid Kit and analyzed using the ACQUITY UPLC I-Class PLUS System and Xevo TQ-S micro Mass Spectrometer.
Both Waters in-house and external CDC DBS materials were extracted and incubated with the Kairos Internal Standard Solution. Extracts were transferred to a 96-well collection plate containing borate buffer and derivatized using the AccQ•Tag Ultra “3X” Derivatizing Reagent. A 2 µL sample extract was injected and quantified using the Kairos Amino Acid Kit Calibrators.
UHPLC-MS/MS analysis successfully quantified 37 amino acids extracted from a single DBS for clinical research, with the remaining 5 reported for semi-quantitative purposes only. Chromatographic separation was performed using an ACQUITY UPLC I-Class PLUS System and a CORTECS™ UPLC C18 Column, followed by detection on a Xevo TQ-S micro Mass Spectrometer (Figure 1).

Experimental
Sample Description
Reagent Kit
Refer to Kairos Amino Acid Kit (p/n: 176004379) and Care and Use Manual (p/n: 720006448) for reconstitution of Kairos Amino Acid Kit Calibrator and Quality Control Sets, Kairos Amino Acid Kit Reagents, Borate Buffer, and AccQ•Tag Ultra “3X” Derivatization Reagent.
The Kairos Internal Standard (IS) was reconstituted using 2 mL of 80:20 MeOH:H2O (80:20 methanol/water v/v) and mixed at room temperature for 10 minutes, ensuring all material was fully dissolved. The contents of the vial were transferred to a volumetric flask and made up to 50 mL using 80:20 MeOH/H20.
Calibrators and QC Sample Preparation
Calibrator and QC samples were prepared fresh on the day of analysis.
- Step 1. Add 75 µL of Kairos IS to a 1.5 mL microfuge
- Step 2. To this add 20 µL of Calibrator/QC sample
- Step 3. Vortex for 5 seconds
- Step 4. Leave samples aside until required (Step 5 below)
DBS Sample Preparation
DBS samples were extracted fresh on the day of analysis.
- Step 1. Punch a 3.2 mm DBS disc into 350 µL collection plate
- Step 2. Add 75 µL of Kairos IS to disc
- Step 3. Seal plate with foil adhesive and incubate at room temperature for 20 minutes, 500 rpm
- Step 4. Add 70 µL of borate buffer containing 0.5 M NaOH to a 350 µL collection plate
- Step 5. Transfer 10 µL of incubated DBS extract/Calibrator/QCs into borate buffer*
- Step 6. Pipette to mix
- Step 7. Vortex AccQ•Tag Ultra “3X” Reagent and add 20 µL to each sample
- Step 8. Seal plate with silicone cap mat
- Step 9. Allow plate to rest at room temperature for 1 minute
- Step 10. Incubate sample plate for 10 minutes, 55 °C, 500rpm
- Step 11. Inject 2 µL on UHPLC-MS/MS System
Note: *On transfer, the DBS sample extracts are translucent and light yellow in the borate solution, Calibrators and QCs extracts are colorless.
Method Conditions
The LC Conditions, Gradient Table, MS Conditions and MRM Parameters are detailed in the Kairos Amino Acid Kit Care and Use Manual (p/n: 720006448).
Data Management and Processing
|
MS software: |
MassLynx™ v4.2 Software (SCN 1042) |
|
Informatics: |
Informatics: TargetLynx™ XS v4.2 Application Manager waters_connect™ Software with QUAN Review Application |
|
Data processing: |
TargetLynx Software (User factor of 6.45 must be applied for quantitation of DBS samples only). |
Results and Discussion
Kairos Amino Acid Kit Performance
No significant carryover was observed from the Kairos Amino Acid Kit Calibrator 6 into subsequent blank samples for all amino acids (detector response ≤20% of LOQ) and no functionally significant carryover was observed on in-house prepared QC High DBS extracted samples into subsequent Endogenous DBS extracts.
Matrix effects were assessed on six independent sources of contrived DBS endogenous samples incubated in triplicate using the Kairos IS Solution. The IS peak area was compared to IS peak area not exposed to DBS matrix no significant ion enhancement or (<±15% compared to control, %CV <10%) was observed, except for histidine.
Linearity was assessed using the Kairos Amino Acid Kit Calibrators on one day (n=4). Regression analysis demonstrated a linear fit using 1/x weighting across the measuring range listed for the 42 amino acids (Appendix Table 4). For each amino acid, the coefficient of determination (R2) was > 0.99 and the %imprecision for each calibrator was ≤15%, except for at the lower limit of the measuring interval (LLMI) ≤20%.
Analytical sensitivity was assessed by analyzing samples prepared in 0.1 M HCl over the range of 0.5–20 µM, replicates of ten per level across five analytical runs. The results are presented in Appendix Table 3. The LOQ for the amino acids analyzed were less than Calibrator 1 measured values except for histidine (9 µM), anserine (20 µM), 3-methyl histidine (10 µM), 1-methyl-histidine (10 µM), and argininosuccinic acid (20 µM).
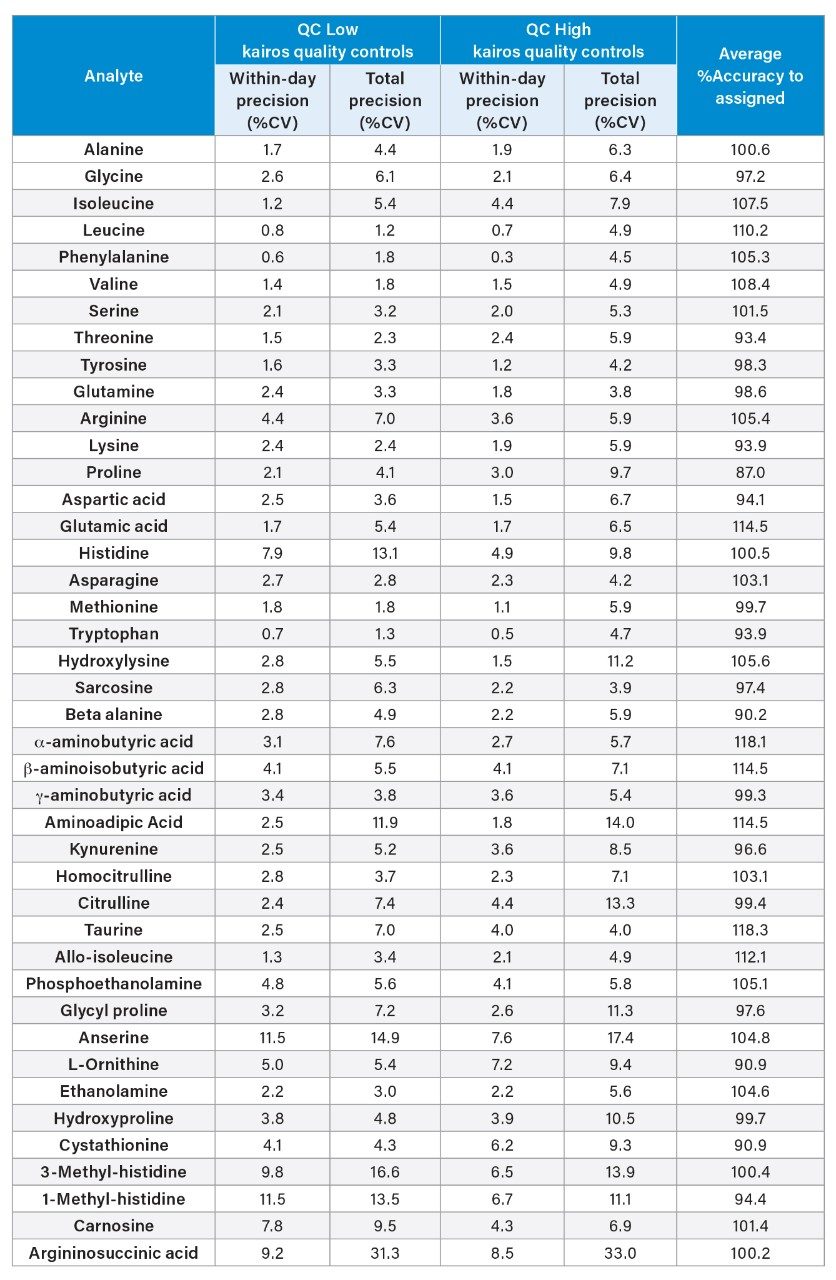
Kairos Amino Acid Kit QC solvent samples (QC LOW and QC HIGH) precision and accuracy were assessed in replicates of five over five analytical runs. Average within day, total precision performance (%CV) and % Accuracy to assigned values were calculated for each analyte. The Kairos QC within-day precision was ≤11.5 %CV and total precision was ≤14.0 %CV except for anserine, 3-methyl-histidine and argininosuccinic acid which exceeded precision goals of ≤15 %CV. The %Accuracy to QC measured values were within ±15% of the assigned values. See Appendix Table 5 for the full summary.
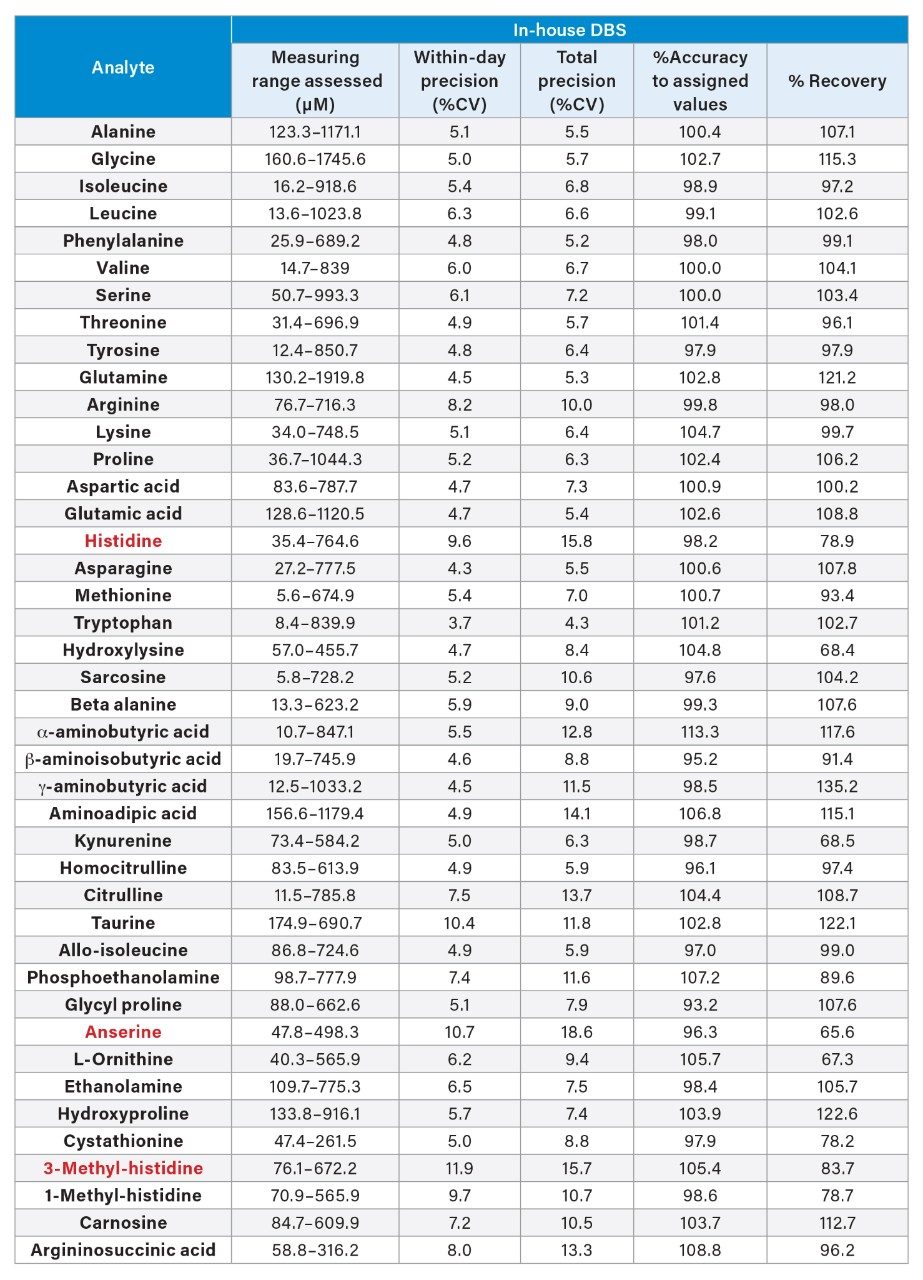
In-House DBS Amino Acid Precision, Accuracy, and Recovery
In-House DBS QCs were prepared (55% Hematocrit) at 5 concentrations (QCs A-E) covering the Kairos Amino Acid Kit measuring range. The in-house DBS QCs were assessed for precision, accuracy, and recovery performance, 5 replicates per QC across five analytical runs (n=25). The average within-day precision and total precision was ≤15 %CV except for histidine, anserine and 3-methyl-histidine (total precision ≤18.6 %CV). %Accuracy to assigned values for all amino acids were <15%.
Recovery was assessed on QC B-E by deducting the endogenous QC A calculated concentrations and results compared to the spiked amino acid amount. Each amino acid recovered within 40–140%, the average recovery ranged from 65.5–135.2%. See Table 1 for the full summary of the data.
A single 3.2 mm DBS punch contains approximately 3.1 µL of blood and a user factor of 6.45 is applied in TargetLynx Software to correct the measurements of amino acids from extracted DBS when quantified using the solvent Kairos Amino Acid Calibrators as described above.
To evaluate method performance, external DBS material from the CDC were assessed for precision and accuracy (5 replicates over 5 analytical runs, n=25) to CDC reported values. Within-day precision ≤6.8%, total precision ≤14.4% and %Accuracy to CDC measurement concentrations were within ±15%. See Table 2 for the full summary.
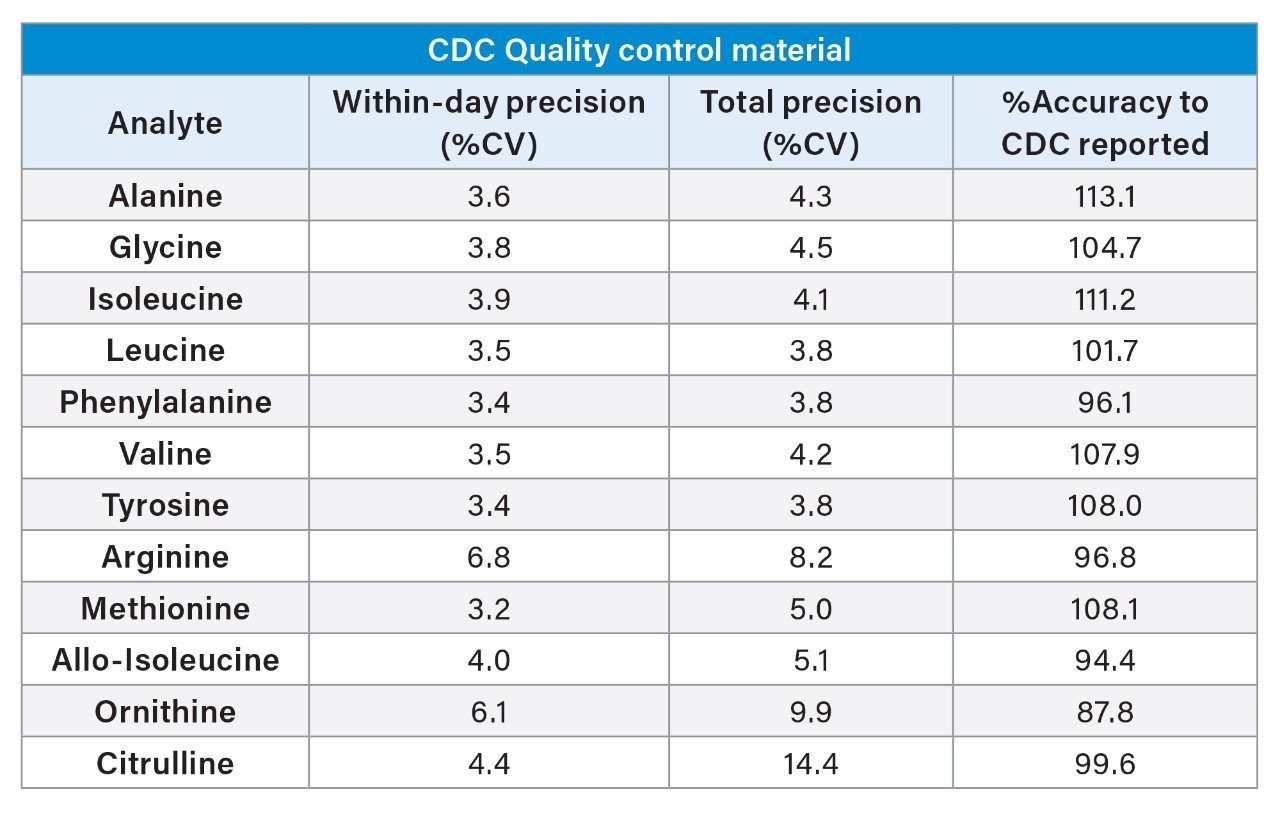
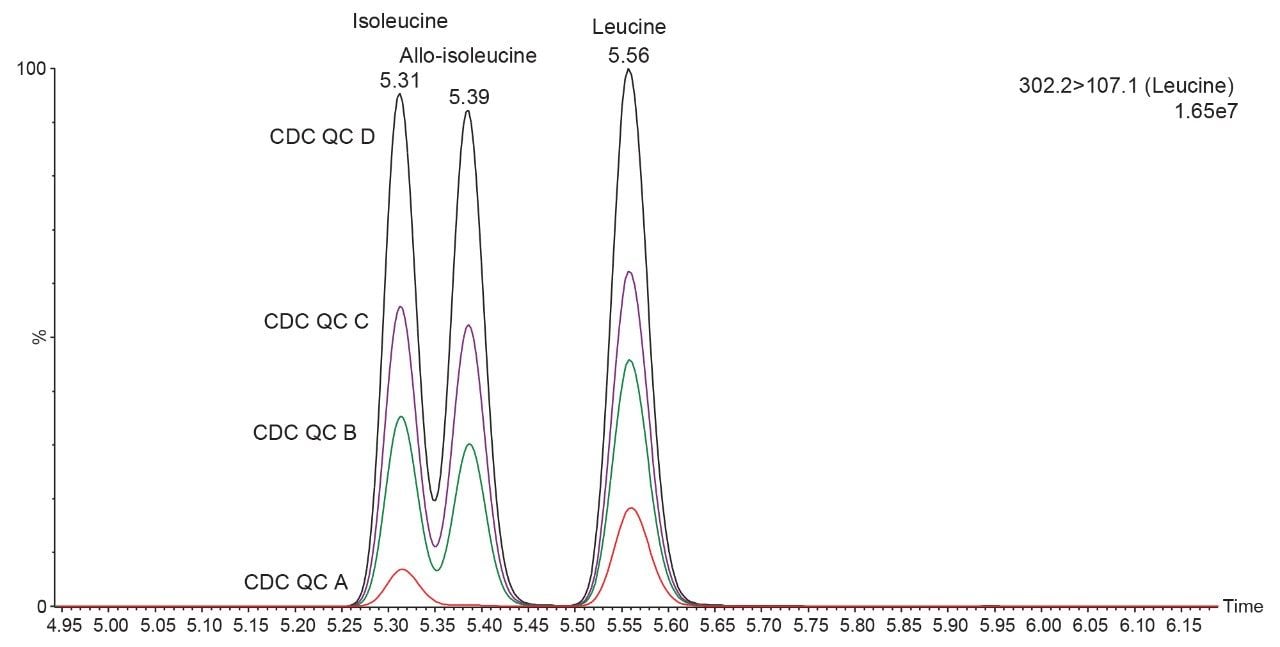
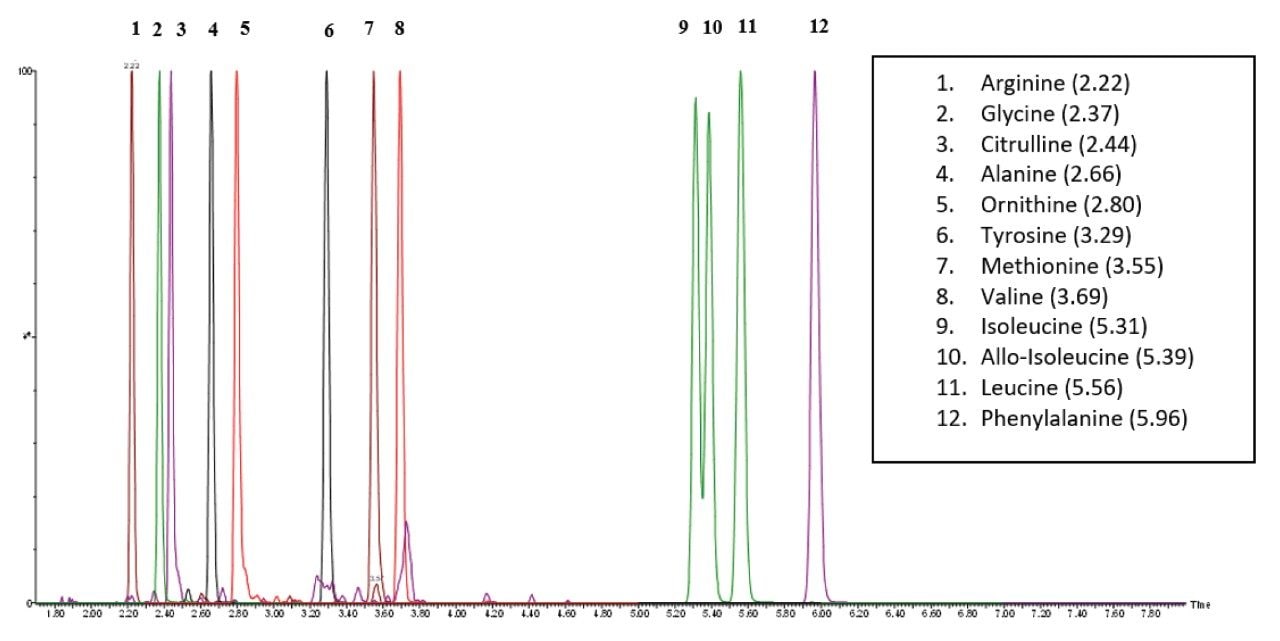
Figure 2 shows chromatographic traces of isobaric leucines (leucine, isoleucine, and allo-isoleucine) in the external DBS material covering 4 QC levels, Endogenous (Red trace), 100 µM (Green trace), 200 µM (Purple trace), and 400 µM (Black Trace). Figure 3 shows the Total Ion Chromatogram (TIC) for the 12 amino acids present in the external DBS material.
Conclusion
The Kairos Amino Acid Kit enables clinical researchers to achieve reliable results with a single, flexible kit that exhibits no significant carryover or matrix effects, and demonstrates good linearity, analytical sensitivity, precision, and accuracy. This method allows for the quantification of 42 amino acids in a single DBS in under 10 minutes using the ACQUITY UPLC I-Class System and Xevo TQ-S micro Mass Spectrometer.
The method eliminates the need for mobile-phase buffers or ion-pair reagents. With the derivatized samples remaining stable. The chromatographic conditions effectively separate isobaric amino acids, ensuring confident peak identification.
The clinical research method demonstrated good precision, accuracy, and recovery for 37 amino acids assessed in the in-house DBS material. The remaining five analytes; anserine, histidine, 1 and 3-methyl histidine and argininosuccinic acid are included; however, they did not fulfill all method performance criteria.
References
Appendix
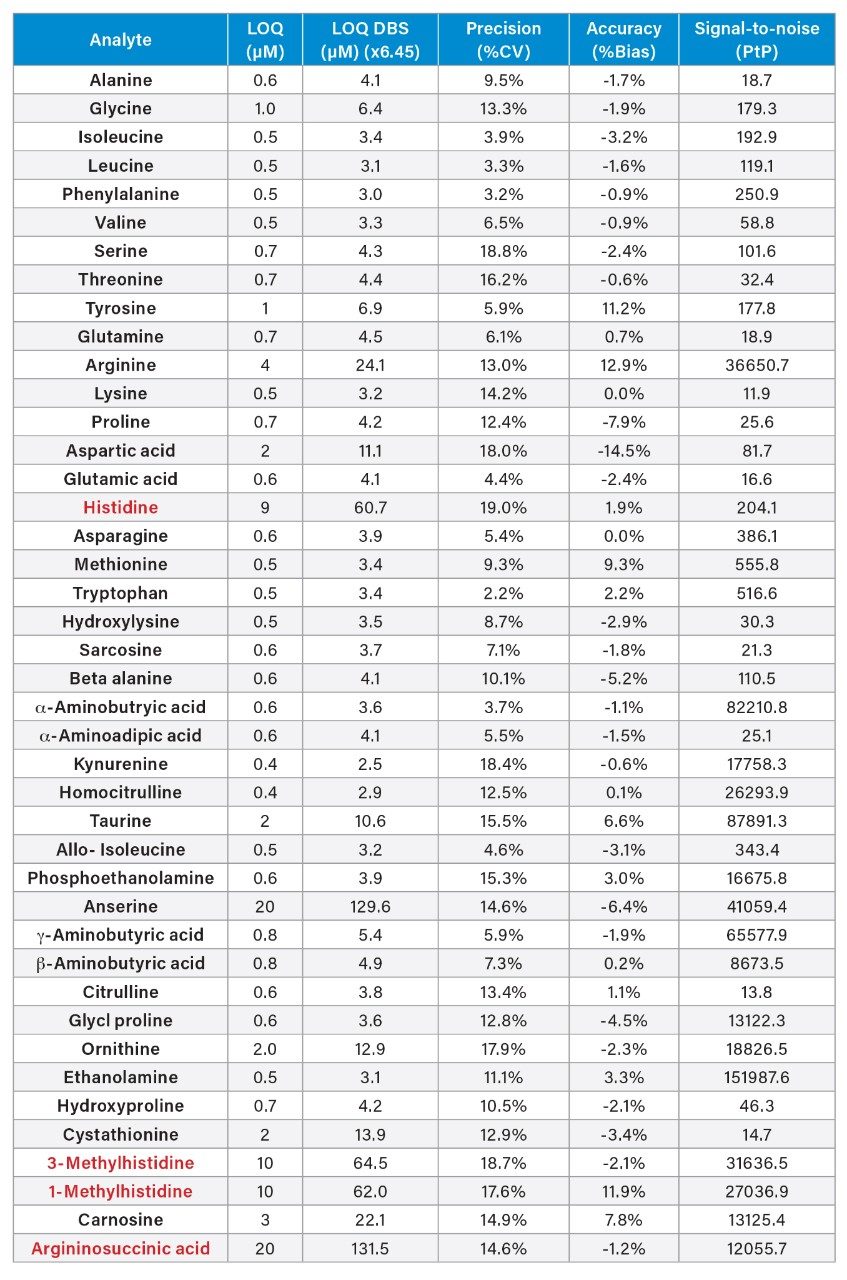
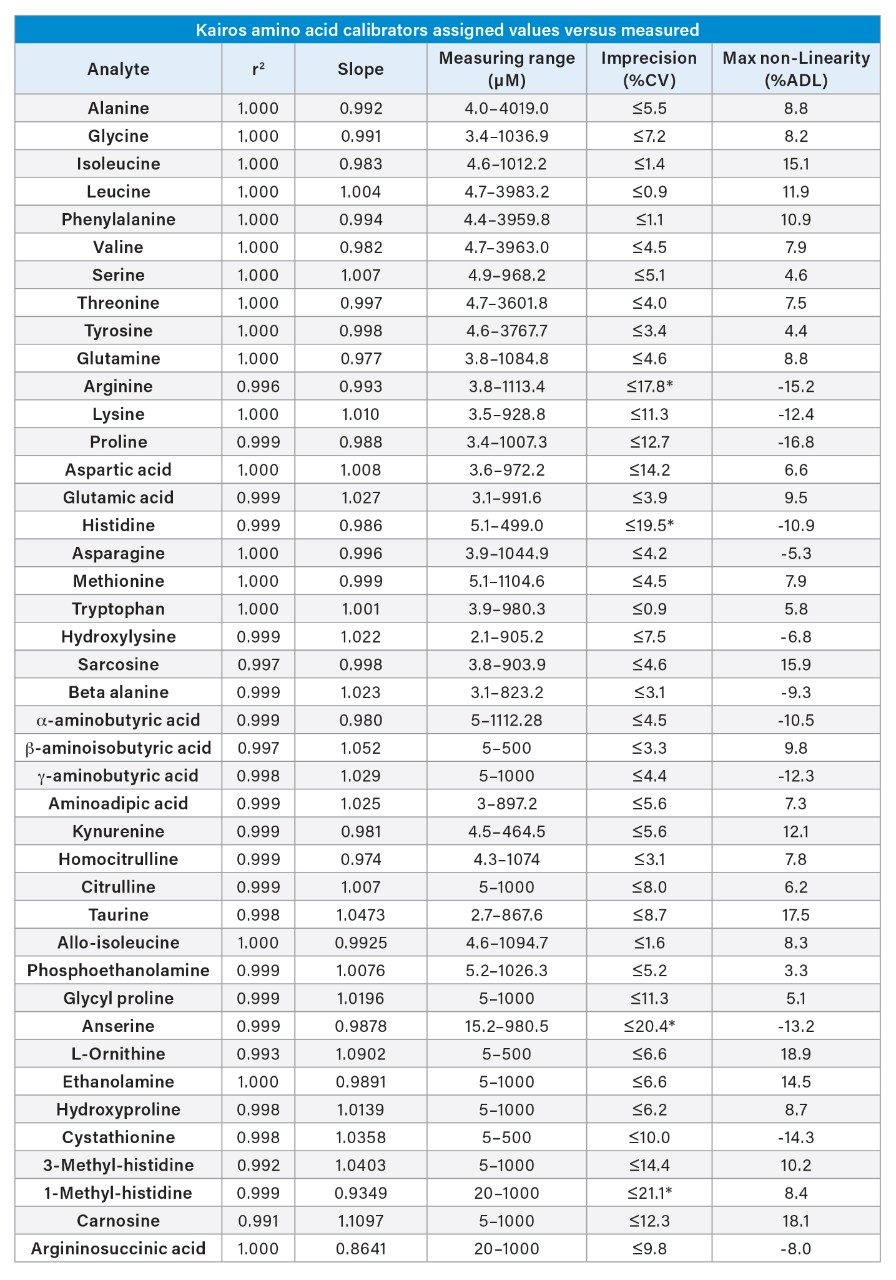
Table 4. Linearity of the Kairos Amino Acid Kit in solution.
*Denotes maximum %CV at LLMI.
Kairos, AccQ•Tag, ACQUITY, UPLC, Xevo, CORTECS, MassLynx, TargetLynx, and waters_connect are trademarks of Waters Technologies Corporation.
720008827, May 2025