Optimizing Sample Preparation Protocols for Extraction of a Panel of PROTACs Molecules From Biological Fluids
Abstract
Benefits
- Systematic method development for PROTACs drugs
- Oasis™ PRiME HLB significantly reduces the amount of phospholipids in the sample
- Oasis WAX™ sorbent provides high recoveries for the pane of PROTACs tested
- Sample extraction from biological fluids can be completely automated using the Andrew+™ pipetting robot and Extraction+™ connected device
Introduction
Drug discovery and development is an ever-evolving scientific discipline, with scientists from academic institutions and pharmaceutical/biotech/biopharmaceutical companies constantly pushing the boundaries. This often results in emergence of novel therapeutic modalities which enable scientists to probe and explore previously undruggable targets. One such recent advancement has been the use of Proteolysis-Targeting Chimeras (PROTACs). PROTACs are heterobifunctional molecules comprising of a warhead that binds to the target of interest, a ligand that recruits the cells protein degradation machinery and a linker that connects these two molecules together.
PROTACs molecules present some unique bioanalytical challenges. These are fragile high molecular weight (900–1200 Da) small molecules which are susceptible to in source fragmentation and non-specific adsorption on plastic, glass, and metal surfaces. In addition, while quantifying these molecules, understanding the distribution of free vs bound vs total can also be important. Developing a robust sample extraction and LC-MS/MS method for the quantification is therefore critical to a successful clinical program.
Here we describe a systematic approach to developing the optimal method for extraction of a panel of PROTAC and its related small molecules from biological fluids.
Experimental
Materials and Methods
|
LC system: |
ACQUITY™ Premier UPLC™ |
|
Vials: |
LCGC Certified Clear Glass 12 x 32 mm Screw Neck Vial, Total Recovery. p/n: 186000384C |
|
Columns: |
ACQUITY UPLC HSS T3 Column, 100 Å, 1.8 µm, 2.1 mm x 100 mm. p/n: 186003539 |
|
Sample preparation: |
Oasis PRiME HLB 96-well µElution Plate (p/n: 186008052) and Oasis Method Development 96-well µElution Plate, (p/n: 186004475) |
|
Column temperature: |
60 °C |
|
Sample temperature: |
4 °C |
|
Injection volume: |
2 µL |
|
Flow rate: |
0.6 mL/min |
|
Mobile phase A: |
Aqueous 0.1% formic acid, 1 mM ammonium formate |
|
Mobile phase B: |
95% acetonitrile 5% water, 0.1% formic acid, 1 mM ammonium formate |
Gradient Table
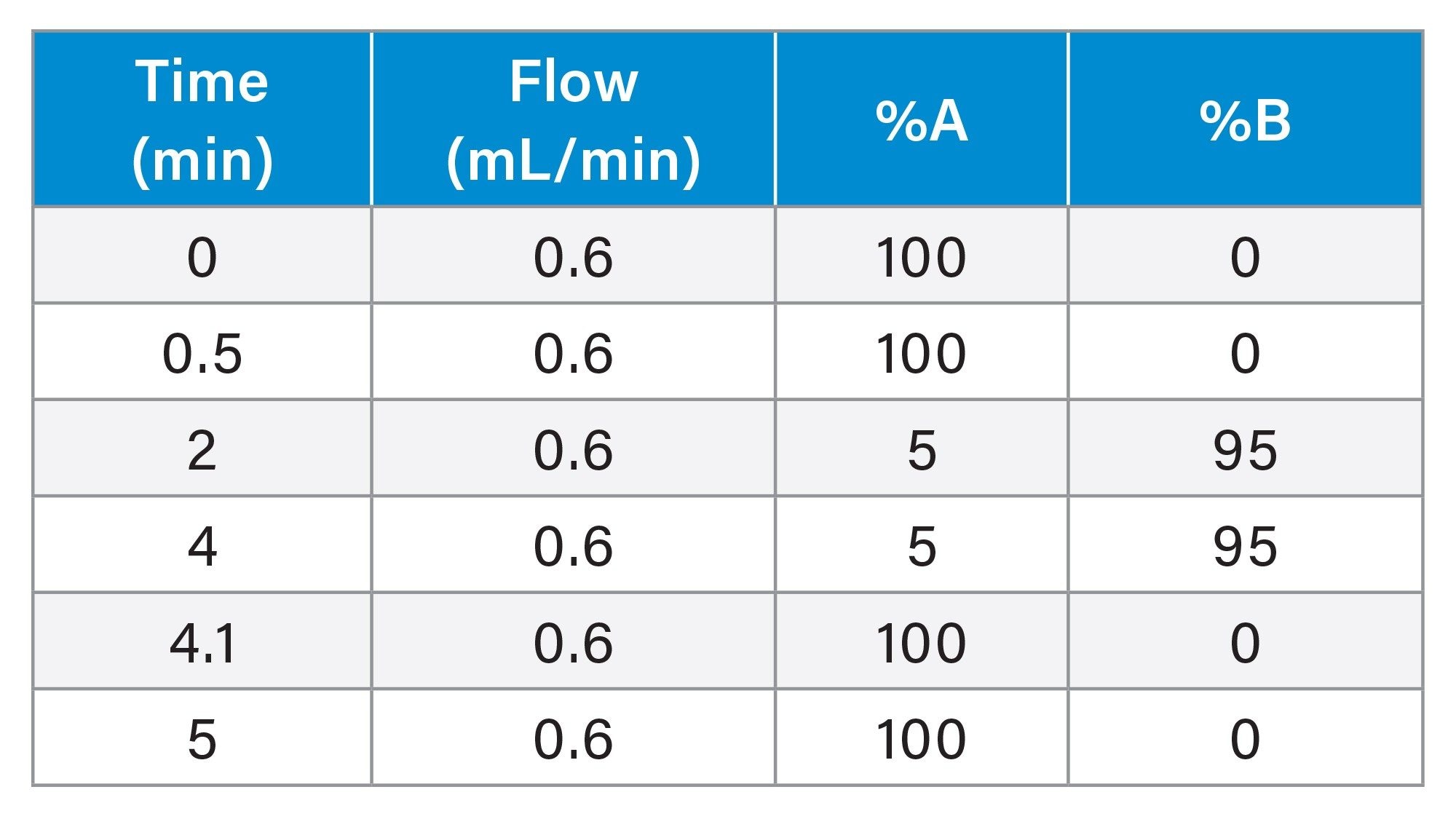
MS Conditions
|
MS system: |
Xevo™ TQ Absolute |
|
Ionization mode: |
Positive ion ElectroSpray Ionization (ESI) |
|
Capillary voltage: |
1 kV |
Data Management
|
Acquisition software: |
MassLynx™ 4.2 Software |
|
Processing software: |
TargetLynx™ XS Software |
Results and Discussion
Quantitative bioanalytical laboratories provide support for multiple programs from discovery, all the way through development and into the clinic. The needs of the assays used to quantify molecules at each stage may differ, based on the requirements of the program. Usually, in discovery settings, the aim is to develop a simple, fit-for-purpose assay, which ideally can be used across multiple analytes in the program to ensure efficiency and ease of operations in a fast-paced environment. For development and clinical assays, the aim is to develop ultra-sensitive assays to help uncover pharmacokinetic and pharmacodynamic insights that may be critical to understanding the clinical pharmacology of the drug candidate. Sample preparation is often the first critical step during the method development process which can significantly influence final assay performance.
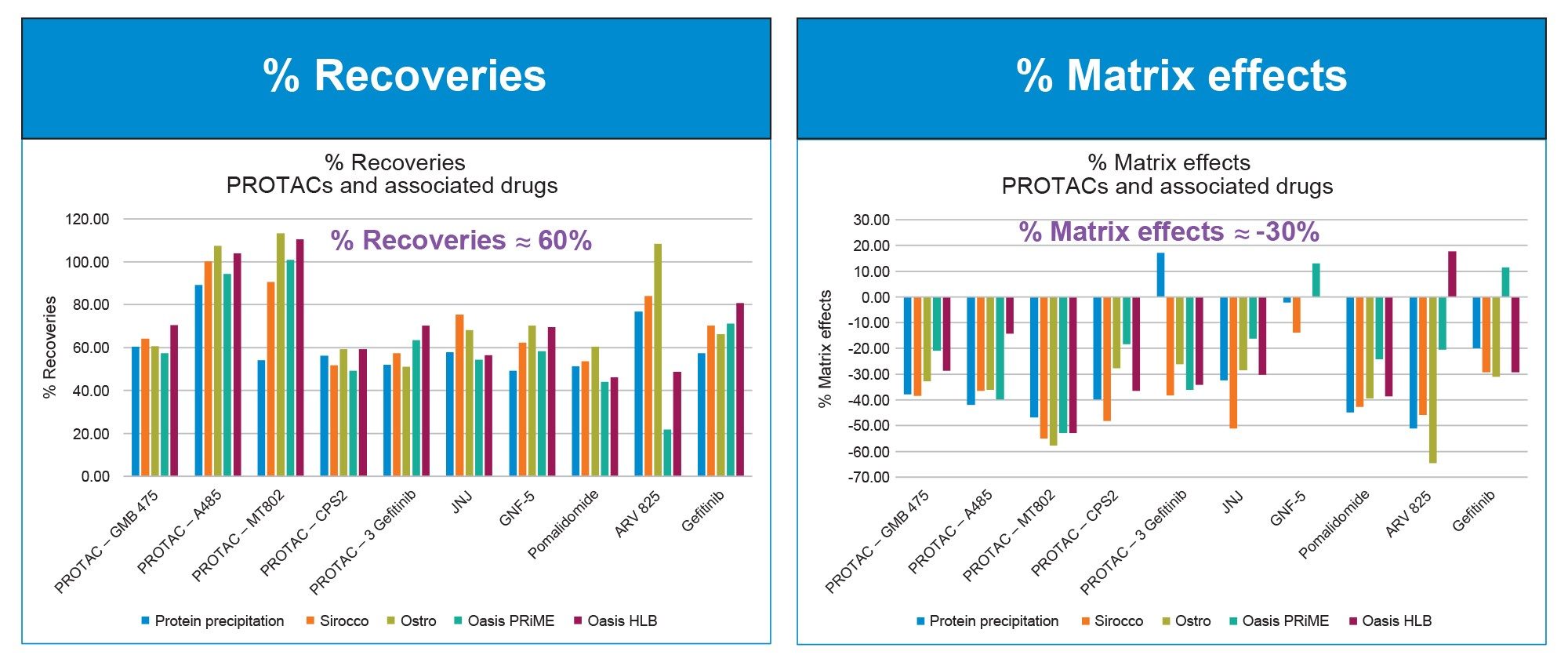
In this study, we screened several commonly used sample preparation techniques to extract a panel of six PROTACs and related small molecule drugs to explore which sample preparation tools might be ideally suited for these analytes during the different stages from discovery to development. We evaluated traditional protein precipitation, Sirocco protein precipitation plate, Ostro protein precipitation and phospholipid removal plate, Oasis PRiME HLB, and Oasis HLB. The primary analytical parameters used for comparison were % Recoveries, % Matrix Effects and % Phospholipid removal.
Using the recommended protocols described in the care and use manuals for each of the above products, we observed that % recoveries for most analytes were very similar (≈60%) to each other (Figure 1A), across all of the sample preparation techniques used. Broadly speaking, the % Matrix Effects were slightly lower across most analytes using the Ostro Phospholipid removal plate, or the Oasis PRiME plate (Figure 1B).
We also looked at normalized % Residual Phospholipids for each of the sample preparation techniques and observed that the Ostro phospholipid removal plate and the Oasis PRiME plate removed ≈98% of the phospholipids, whereas the Oasis HLB sorbent removed ≈88% of the phospholipids (Figure 2). This might partly explain the lower matrix effects observed for some of the analytes using the Oasis PRiME plate.
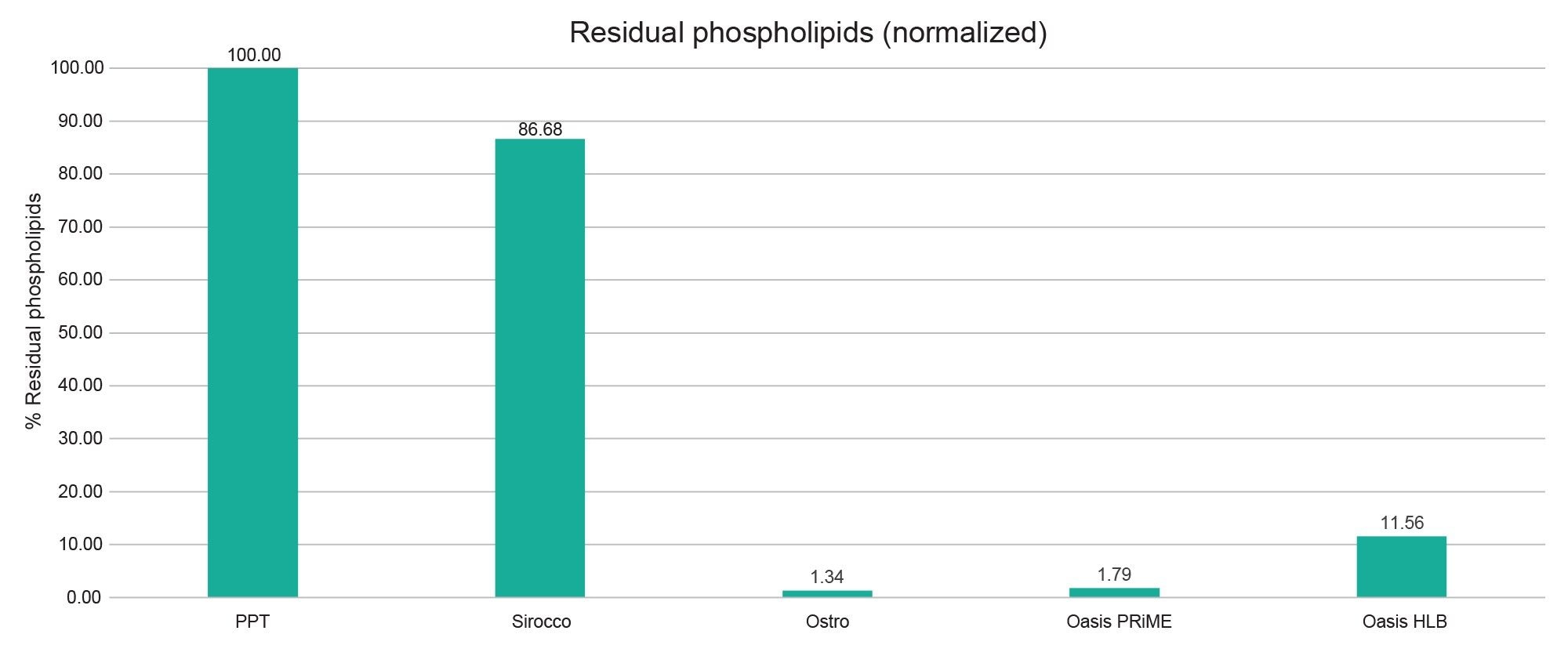
In discovery settings, where a generic fit-for-purpose assay is desired, using Oasis PRiME plate provides acceptable recoveries across the analytes tested, while significantly reducing the residual phospholipids, resulting in lower matrix effects, leading to a more robust assay in the long term. Additionally, this may also result in cleaner LC-MS system, which also reduces potential downtime.
As the drug candidates move into development, and ultimately into the clinic, the sensitivity of the assay becomes extremely important, as labs are trying to do more with limited sample volumes. For our panel of PROTACs and related small molecules, we wanted to explore ways of increasing the % recoveries to increase the sensitivity of the assay. We used the Oasis Mixed mode sorbent selection plate along with the Oasis 2x4 Method development protocol described in the care and use manual. Following the method development protocol illustrated in Figure 3, we observed that the % recoveries for all but one of the PROTACs molecules was >90% (Figure 4A) and the % recoveries for all but one 1 related small molecule drug was ≈100% in the WAX neutral fraction eluate (Figure 4B).
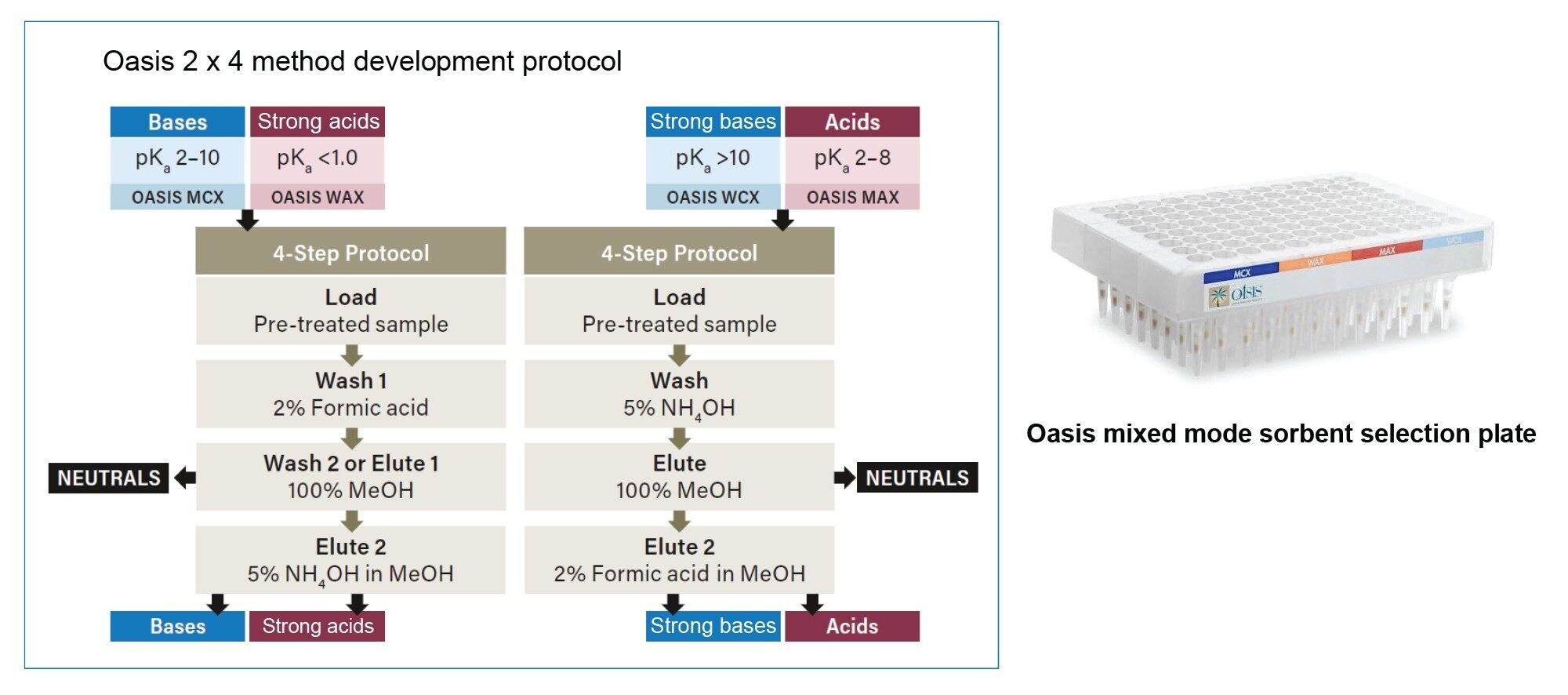
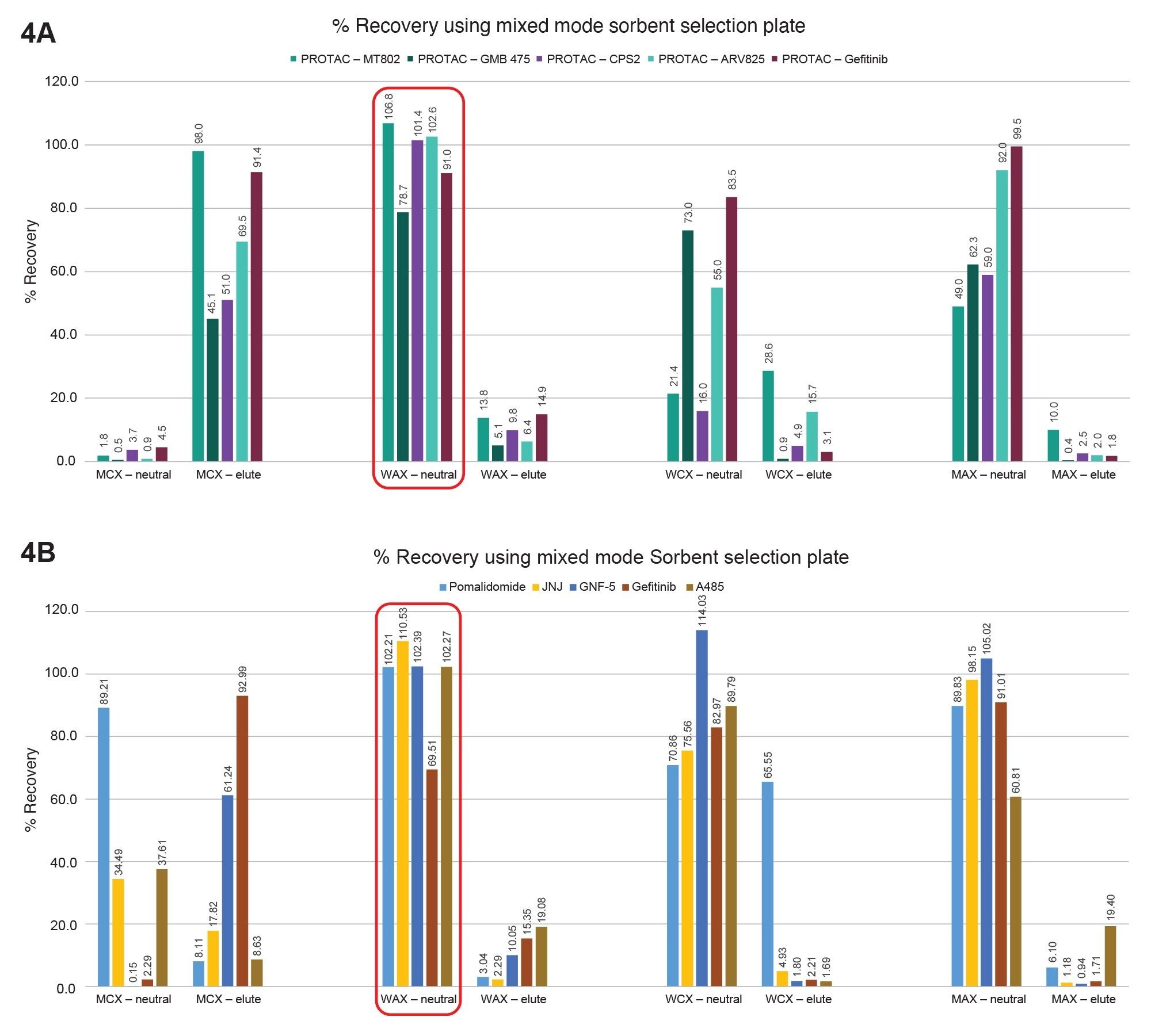
Figure 4. A) % Recoveries for PROTACs drugs using Oasis Mixed mode sorbent selection plate.
Figure 4. B) % Recoveries for related small molecule drugs using Oasis Mixed mode sorbent selection plate.
Conclusion
PROTACs are a new class of therapeutic modalities which have shown promising results in the clinic. The structure of PROTACs requires some unique considerations while developing appropriate bioanalytical assays for these molecules. For the panel of PROTACs and related small molecule drugs tested here, we have demonstrated that:
- Oasis PRiME HLB provides adequate recoveries with >98% phospholipid removal resulting in cleaner samples, making it appropriate for generic fit-for-purpose assays
- Oasis Mixed mode (WAX) provides the highest recoveries, making it ideally suited for assays requiring ultimate sensitivity
720008599, November 2024