This is an Application Brief and does not contain a detailed Experimental section.
Chromatographic columns, if maintained properly, can be used for hundreds to thousands of injections. However, no matter how well maintained, a column will eventually lose performance. A column with poor performance can produce failing results and lead to costly rework. In order to track column lifetime, routine injections of a performance standard can be used. In this work, a Quality Control Reference Material (QCRM) is used to monitor column performance throughout testing of protein precipitated plasma.
Ensuring data quality in routine bioanalytical analyses can be a challenging endeavor. The use of system suitability injections along with pass/fail criteria is a good starting point that has been used in the pharmaceutical industry for many years. However, system suitability injections are usually run before samples and only tests if the system is working properly at the beginning of the analysis. Imagine an analyst must run a full 96-well plate of samples during their shift. While the system suitability may pass at the beginning of the analysis, the system could “fail” part way through leading to inaccurate results with no indication of a failure until data processing. Even then, due to the challenging nature of the samples, the poor results seen in data processing could be caused by a variety of factors and not necessarily a system failure.
By performing system suitability injections more frequently and comparing the results, the analyst can confirm the system was working properly throughout the data set. This adds a level of reliability in the data and eliminates LC system malfunction as a cause for any poor results. Performing routine checks of the system also allows the column performance to be tracked, allowing the analyst to predict when a column starts to fail. The frequency of injections should be determined at the discretion of the analyst or laboratory manager. The data from a failed injection can also be used to help troubleshoot the system to reduce downtime. Lastly using a standard to check system performance can be done independently of the assay being performed, allowing the same standard and conditions to be used with a variety of assays and systems. In Figure 1, an example of bracketing sample analysis with a standard injection is shown.
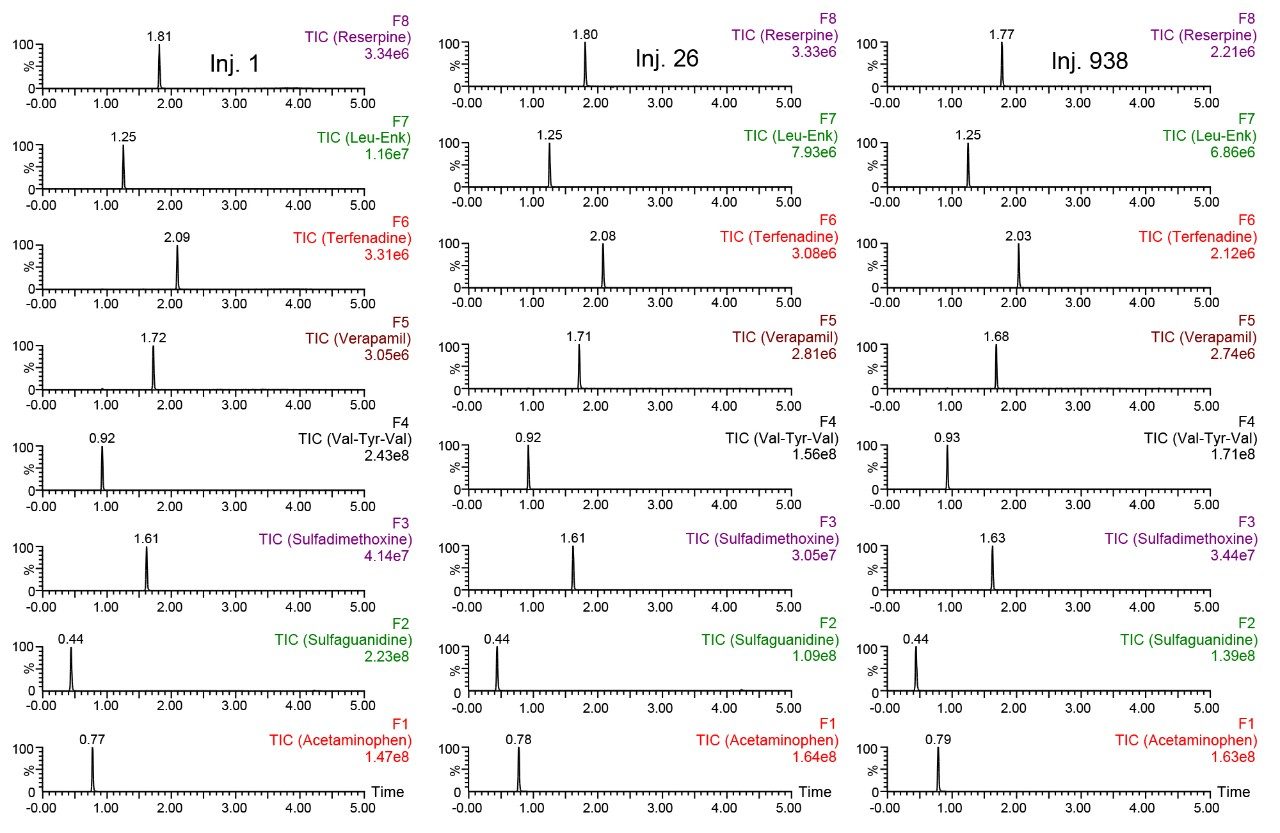
The standard used in this work is the Quad LCMS QC Reference Material (QCRM), designed to test LC-MS systems. The standard contains a variety of analytes which ionize in both positive and negative mode and employs generic LC solvents and conditions. The standard was injected every 25 samples of precipitated plasma for over 900 injections. This frequency was determined by the analyst and may not be appropriate for all labs. Prior to starting the test, the QCRM was used to get a benchmark of system performance which will be used to troubleshoot the system if it fails. By employing all these checks, the analysis of the plasma is performed with a sense of certainty, that whatever results are seen are accurate and not caused by system malfunction while also checking column performance.
720007034, October 2020