For research use only. Not for use in diagnostic procedures.
This application note describes Ostro Pass-through sample preparation method for phospholipids is a simple and reproducible method.
The number and amount of the phospholipid species observed in plasma by this method compares very well to those observed by the standard extraction method (Bligh and Dyer), with the Ostro Pass-through Plate having significant benefits in recovering LPIs and PGs.
The common goals of many “omics” are to identify disease molecular markers and to understand mechanisms of disease which can then suggest possible drug targets. Typically, circulatory body fluids are studied as they have significant contact with affected organs and tissues, and so are sensitive to the body’s specific responses to a pathological condition.
In lipidomic analysis, blood plasma phospholipids are usually studied following solvent extraction.1,2 Solid-phase extraction techniques have also been used in the past. Aminopropyl silica columns were used to analyse lipids, but they either focussed more on fatty acids,3 or were class selective depending on the elution conditions used.4
The Ostro 96-well Sample Preparation Plate is a pass-through device designed to capture and remove the highly abundant phospholipids as part of sample preparation for the analysis of small molecules during routine bioanalysis. In the generic protocol, the lipids are retained by the Ostro Pass-through Plate, and no attempt is made to release them. Modifying the loading and elution conditions allows this same affinity to be exploited for both selective enrichment of certain classes or for total phospholipid extraction.
|
Column: |
ACQUITY BEH HILIC 1.7 μm, 2.1 x 100 mm |
|
Column temp.: |
30 °C |
|
Mobile phase A: |
Acetonitrile/water (95:5) with 10 mM ammonium acetate, pH 8.0 |
|
Mobile phase B: |
Acetonitrile/water (50:50) with 10 mM ammonium acetate, pH 8.0 |
|
Gradient: |
0–20% B for 10 min |
|
Flow rate: |
500 μL/min |
|
Injection volume: |
3.0 μL, partial loop |
|
Ionization mode: |
ESI, +/- switching |
|
Capillary voltage: |
3.0 kV |
|
Desolvation temp.: |
450 °C |
|
Desolvation gas: |
1000 L/hr |
|
Source temp.: |
150 °C |
|
Collision cell pressure: |
3.6 x 10-3 mBar |
Human plasma was obtained either through volunteers at the Centre for Life Sciences, National University of Singapore, or from a commercial sample (Sigma, p/n P9523).
Note: Take care to ensure all the pipette tips are properly positioned in the sample wells to prevent any loss or mixing of samples.
For comparison, aliquots of the same human plasma were extracted using the Bligh and Dyer method.1
Due to the large dynamic range in intensities observed between the phospholipid classes, the samples were analysed at two concentrations; undiluted and diluted 1/100 with acetonitrile. A custom MRM method was used with positive and negative switching and MRMs timed to coincide with the elution times of specific lipid classes1. A summary of this method is shown in Table 1 below.
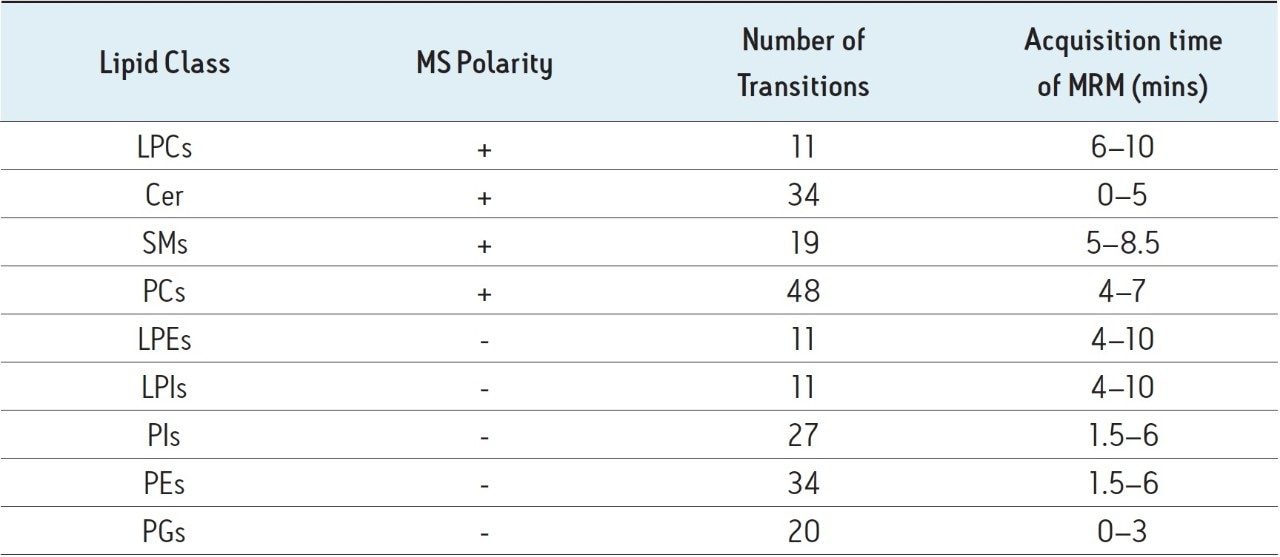
Intensities for each species were automatically extracted as area counts using a pre-programmed TargetLynx method. A mean intensity threshold of 1000 area counts was applied to the data.
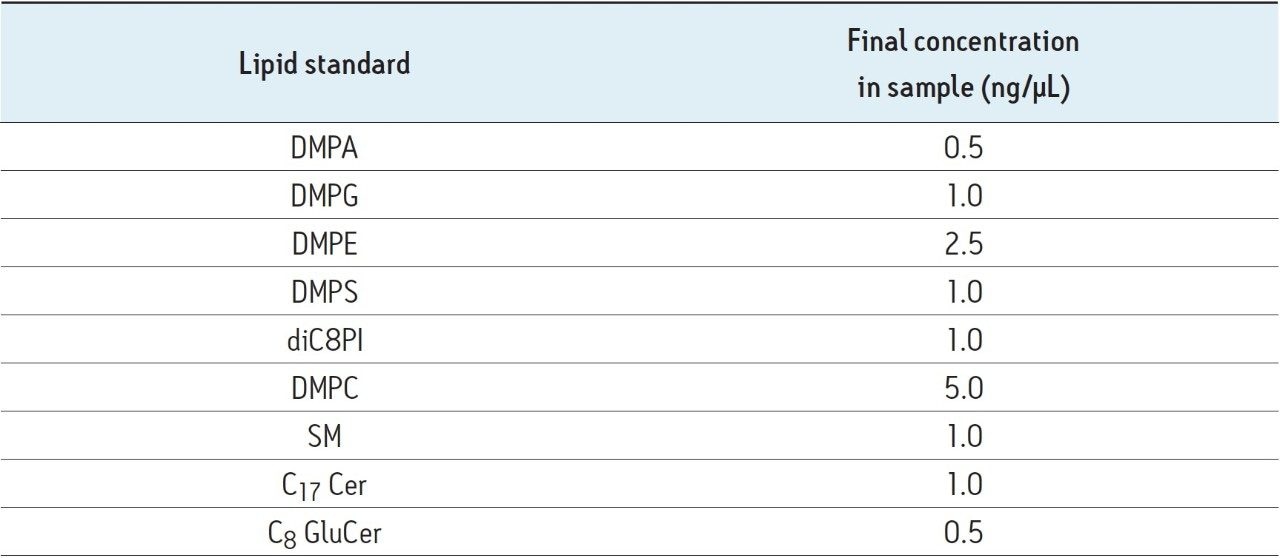
As an experimental control when comparing the two sample preparation methods, exogenous lipids were spiked into the samples immediately prior to analysis. These were used for normalisation prior to calculating the relative extraction efficiency of a method or fraction. The spiked lipids and their concentrations are shown below in Table 2.
Extractions were carried out on mixed volunteer human plasma in duplicate, with analytical duplicates. A threshold of 1000 counts from the appropriate MRM must be exceeded for the detection of a lipid. Peak areas for each lipid species were normalized as a ratio to a post extraction internal spike of an exogenous lipid of the same class. These ratios were then averaged across replicates in the same fraction/method to give a single normalized value for each lipid in that fraction. The values for lipids from the Ostro Pass-through and eluate fractions were divided by the values for the same lipids from the Bligh and Dyer method to give an extraction efficiency expressed as a ratio for each lipid. The average extraction efficiencies of each class are shown in Table 3.
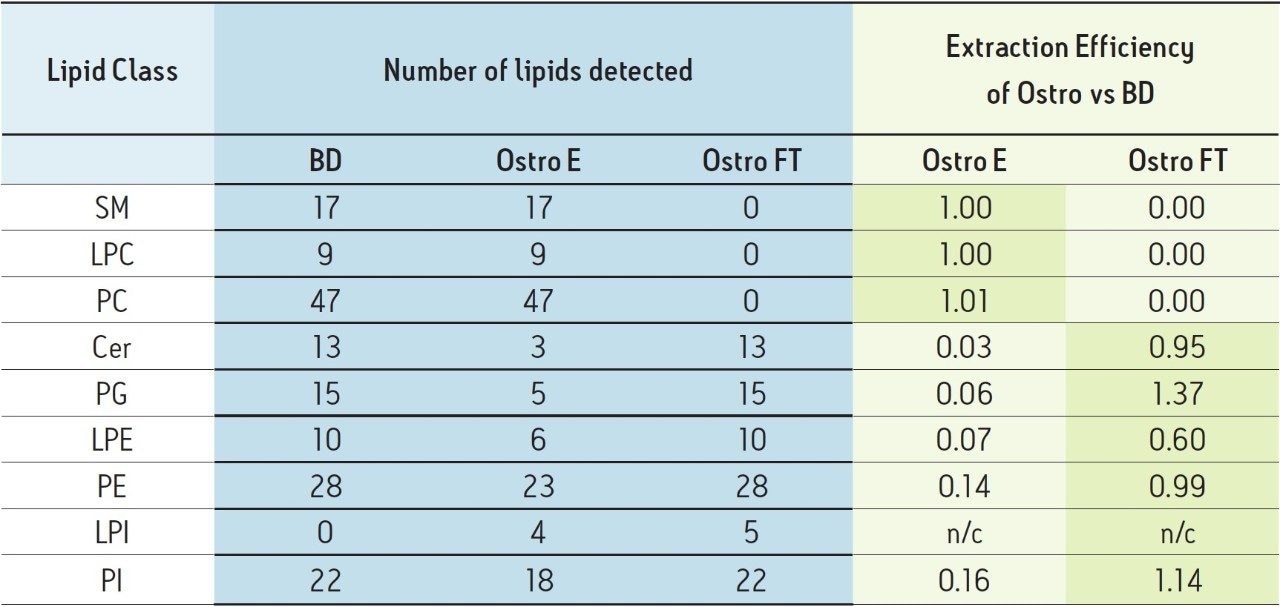
Sphingomyelins (SMs), phosphatidylcholines (PCs), and lyso-phosphotidylcholines (LPCs) are completely retained on the Ostro plate and only appear in the eluate. The same phospholipid species were observed by both extraction methods at the same intensities.
Ceramides (Cer) and phosphatidylglycerols (PGs) appear to have little affinity for the solid phase under these conditions and are found in the flow through fraction, with very small amounts retained and recovered in the eluate fraction. While the same PG lipids were identified, extraction using the Ostro Pass-through 96-well Plate appears to increase the area counts of the PGs compared the Bligh and Dyer method. Phosphatidylethanolamines (PEs) and phosphotidylinositols (PIs) follow a similar behaviour but slightly more lipid is retained by the Ostro plate and appears in the eluate, however, the extraction efficiency appears to be the same as the Bligh and Dyer method. The same lyso-PE (LPE) species are observed by both extraction methods, but it appears that the recovery from the Ostro Pass-through Plate is about 60% of the Bligh and Dyer method.
Extraction of Phospholipids from Plasma using Ostro Pass-through Sample Preparation 5 Extraction of lyso-phosphatidylinositols (LPIs) on the other hand is greatly enhanced by Ostro sample preparation, with 5 additional lipid species being observed using the Ostro Pass-through Plate than by the Bligh and Dyer method. The majority of the LPI (90%) does not bind to the Ostro Pass-through Plate.
In general, SMs, PCs and LPCs bind to the Ostro Pass-through Plate and are only eluted with 4.5:4.5:1 Chloroform:Methanol: Triethylamine. All other classes have minimal interaction with the plate under the conditions used and pass through in the flow through fraction.
The Ostro Pass-through Plate was used to extract 14 aliquots of commercially available human plasma. The flow through (FT) and eluate (E) fractions were analysed separately in triplicate for each extraction. Percentage relative standard deviations (%RSDs) of the area counts were calculated for each lipid species across all replicates in the data set and listed in Table 4.
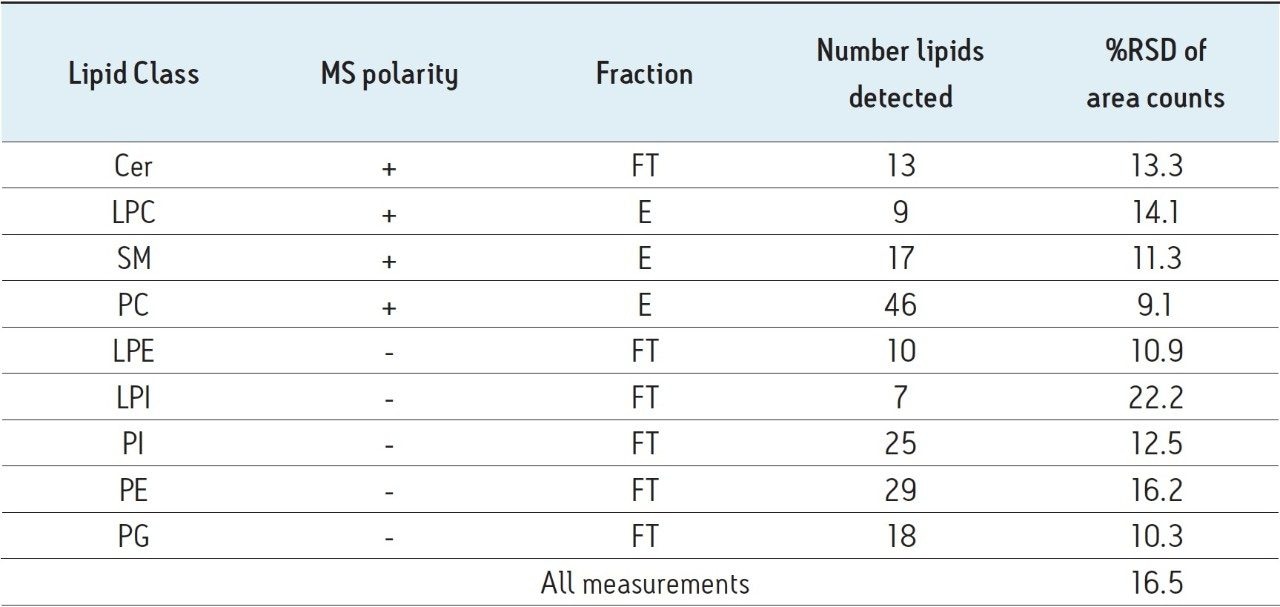
The %RSD shown are influenced by ion intensity, with the less abundant classes contributing more to the overall error in this experiment. The error of the analytical (LC-MS) method has been shown to be 5% at the same intensity threshold.5
The Ostro Pass-through sample preparation method for phospholipids is a simple and reproducible method.
The number and amount of the phospholipid species observed in plasma by this method compares very well to those observed by the standard extraction method (Bligh and Dyer), with the Ostro Pass-through Plate having significant benefits in recovering LPIs and PGs.
This technique is suitable for both small scale and large scale lipidomic studies. As the Ostro Pass-through Plate uses a 96-well format and requires no centrifugation, it can potentially be automated with a laboratory robotics system, which cannot be done with the liquid extraction method. The Ostro Pass-through Sample Preparation Method also uses significantly less solvent than liquid extraction methods.
This technique can be used either as a general all-class extraction method for phospholipids, or it can be used selectively. For example, analyzing only the flow-through fraction allows low abundance species to be studied without dynamic range and ion suppression issues caused by the highly abundant PCs.
720004201, November 2014