
This application note shows the combination of UPC2 and tandem quadrupole mass spectrometry provides for a means to produce fast, high resolution separations of chiral compounds in the DMPK laboratory.
The separation and detection of GSK1322322, a novel antibacterial agent,and three associated stereoisomer metabolites was investigated utilizing UltraPerformance Convergence Chromatography (UPC2) coupled with tandem quadrupole mass spectrometry. This separation was attempted on various other platforms with no success (UV, chiral HPLC, mass spec).
There have been numerous examples of metabolism creating new centers of chirality in new chemical entities (NCEs). This chiral inversion can have pharmacokinetic, pharmacodynamics, and safety consequences.1-3 Therefore because of the heightened awareness of stereoselective metabolism, there are growing government requirements and regulations in chiral metabolite identification and quantification during drug development.
Recent advances have potentially opened the door for the use of UPC2-MS/MS in routine bioanalysis with chiral entities, in a regulated environment. UPC2 applies the performance advantages of UPLC to supercritical fluid chromatography, using supercritical carbon dioxide as the major mobile phase. The results presented here explore the robustness of this technique and its application to clinical study samples in the quest to investigate in vivo chiral inversion.
|
System: |
ACQUITY UPC2 |
|
Column: |
Chiral Pak AD-H, 5-μm, 4.6 x 150 mm |
|
ABPR pressure: |
2750 PSI |
|
Column temp.: |
40 °C |
|
Sample temp.: |
Ambient |
|
Injection vol.: |
5 μL |
|
Flow rate: |
3 mL/min |
|
Mobile phase: |
CO2/isopropanol with 0.4% diethylamine (80/20) |
|
Run time: |
10 minutes |
|
MS system: |
Xevo TQ-S |
|
Ionization mode: |
ESI + |
|
Acquisition mode: |
MRM |
|
Capillary voltage: |
4 kV |
|
Collision energy: |
30 V |
|
Cone voltage: |
25 V |
MassLynx 4.1 Software
GSK1322322, GSK1343981, GSK1785312, and GSK1784667 were extracted from 100 μL human plasma by protein precipitation using acetonitrile containing [2H2 13C2] –GSK1322322 as an internal standard, followed by derivatization with camphanic chloride (1 mg/mL in acetontrile) for 15 minutes at 37 °C. Extracts were analyzed by UPC2-MS/MS using an electrospray interface and multiple reaction monitoring (MRM).
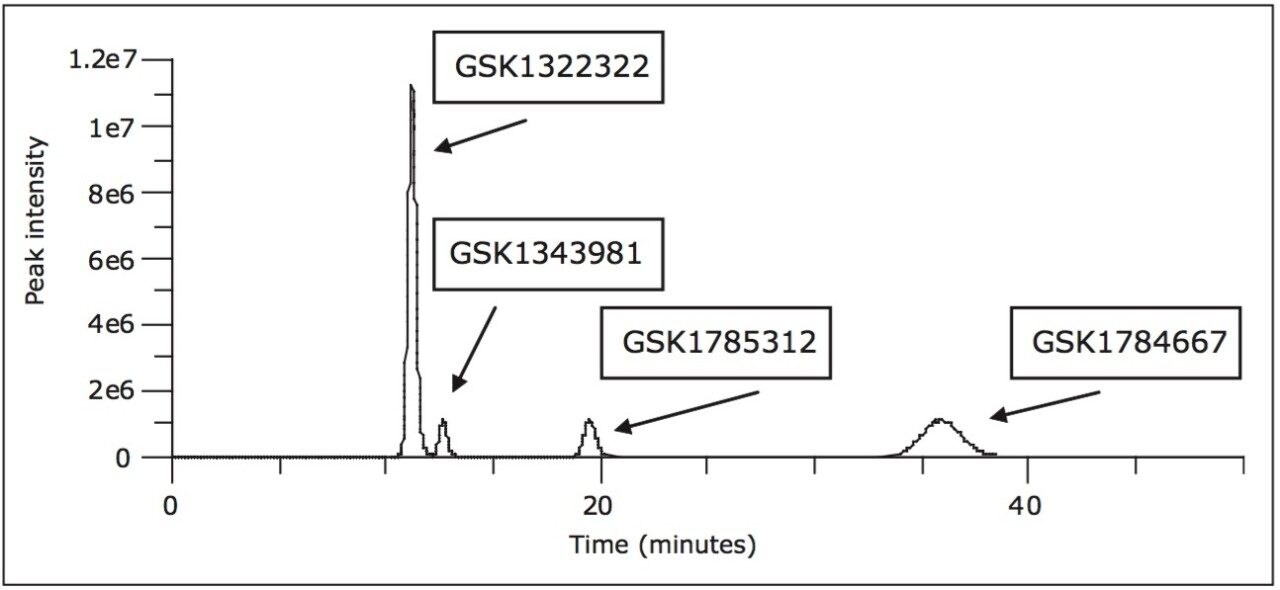
As stated in the introduction, the separation of GSK1322322 and associated stereoisomer metabolites was attempted on a variety of other platforms. One such method that was produced was a 45-minute chiral HPLC-UV method using heptane/ethanol/DEA and formic acid. However this method was neither compatible with nor ideal in a mass spectrometer-based DMPK bioanalytical environment due to the long run time and the organic mobile phase solvents required for the separation. The separation further produced wide peak widths on the order of 2- 5 minutes at the peak base. Figure 1 illustrates the separation that was produced by this method.
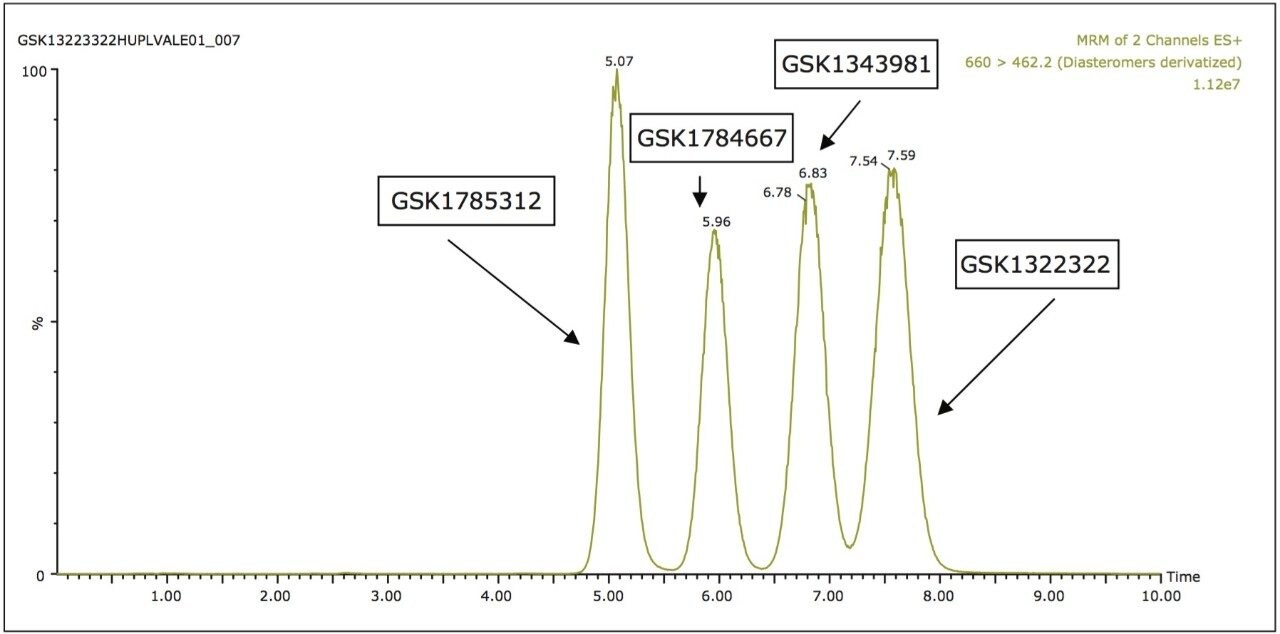
Due to the undesirable attributes of the normal-phase separation and the inability for reversed-phase to resolve all of the components, the analytes were then analyzed by UPC2-MS/MS. As can be seen in Figure 2, the UPC2-MS/MS produced a separation with reduced analysis time with much improved peak shape with near baseline resolution of all analytes.
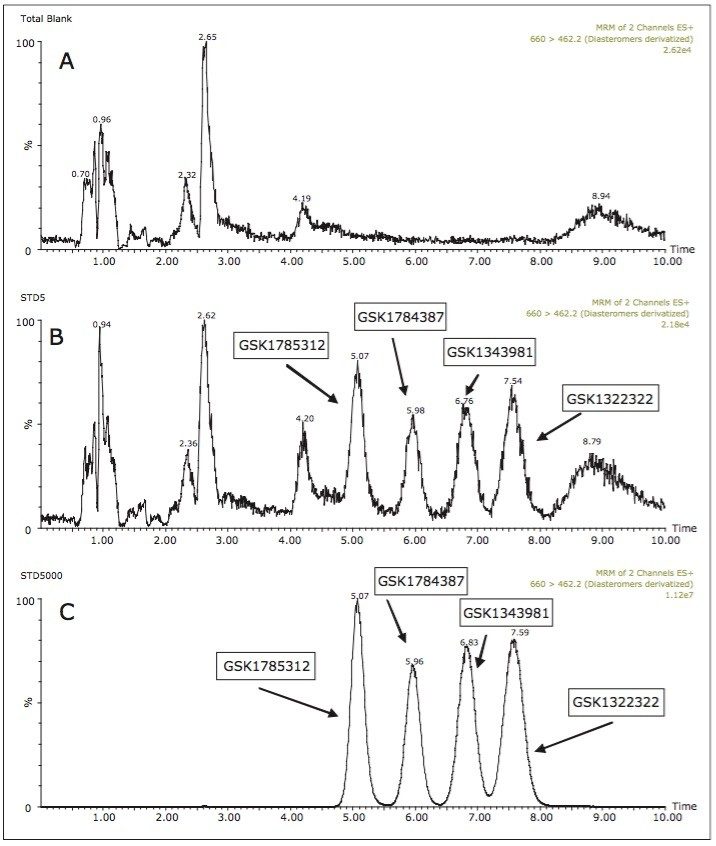
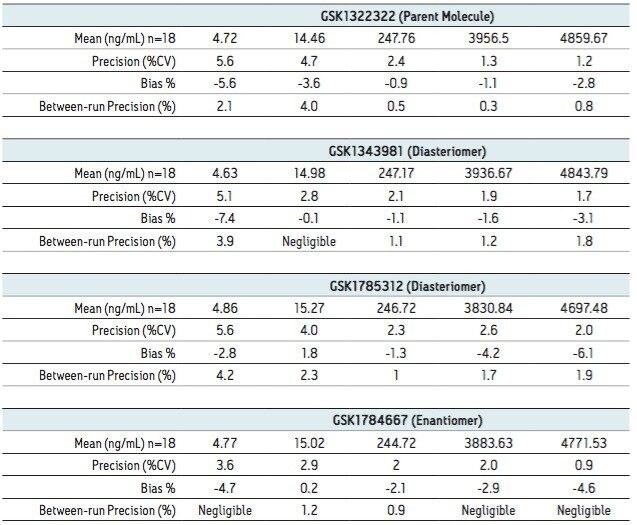
Samples from pooled preclinical and clinical plasma samples where then analyzed using the UPC2-MS/MS method over a concentration range of 5 to 5000 ng/mL. Figure 3 shows the plasma blank along with the LLOQ and ULOQ for all of the four analytes under investigation. A three-day validation study was then performed to assess the precision and accuracy of the method. Table 1 shows the results of the validation study. The results from assay validation show that the method is rugged, precise, accurate, and well-suited to support the analysis of plasma samples.
UPC2 coupled with tandem quadrupole mass spectrometry was successfully applied in the investigation of stereoselective metabolism in a DMPK environment.
The UPC2-MS/MS method was successfully validated over a threeday period for the parent compound GSK1322322 and the three diastereomeric metabolites from pooled clinical and preclinical dog plasma samples.
720004848, November 2013