This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates that the Xevo TQ-XS quadrupole Mass Spectrometer used for quantification of fluticasone propionate (extracted from human plasma) achieved excellent sensitivity and dynamic range, demonstrating its suitability for bioanalytical quantification.
Demonstrate sensitive and robust quantitative performance of the Xevo TQ-XS mass spectrometer to achieve sub-pg/mL detection for fluticasone extracted from plasma.
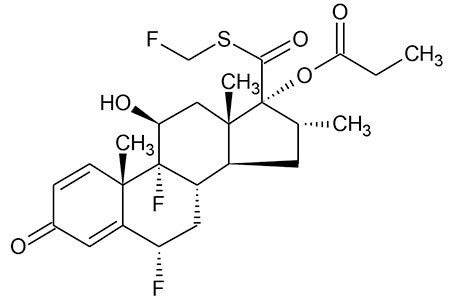
Fluticasone propionate (Figure 1) is a potent synthetic trifluorinated corticosteroid with anti-inflammatory activity and is used in the treatment of asthma.¹ Due to its mechanism of action and route of administration, via nasal inhalation, circulating plasma concentrations are low, in the pg/mL range, making accurate quantification challenging.² This work presented herein, highlights a simple and selective solid-phase extraction (SPE) sample preparation, UPLC chromatographic separation, and tandem quadrupole mass spectrometer (MS) with Unispray ionization for sensitive and robust quantification of fluticasone propionate from plasma. This method achieves lower limits of detection (LODs) of 0.2 pg/mL with a linear dynamic range from 0.2-20 pg/mL, extracted from 600 µL of plasma.
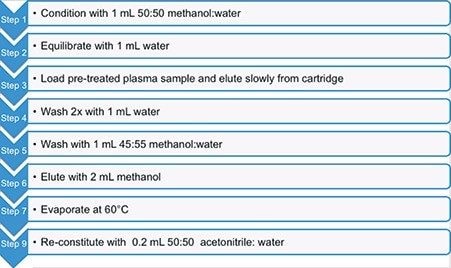
For MS analysis, a multiple reaction monitoring (MRM) experiment was performed on the Xevo TQ-XS. Chromatographic separation was achieved using an ACQUITY UPLC I-Class System and ACQUITY UPLC BEH Phenyl 1.7µm Column (p/n 186002885). Fluticasone plasma samples (0.2 to 20 pg/mL) were prepared using commercially available human plasma. Calibration curve standards were prepared in duplicate to check the reproducibility, while six replicates were prepared for the QC and blank (non-spiked) plasma samples. No internal standard was used. A 600 µL aliquot of each of the prepared plasma samples was pre-treated with 2% formic acid in water and mixed. The pre-treated plasma sample was extracted using the Oasis MCX Cartridges and extraction protocol shown in Figure 2. Following the extraction, 20 µL of extracted sample was injected for LC-MS/MS analysis.
Using this SPE UPLC-MS method, quantitative performance was excellent. SPE recovery for fluticasone propionate, using the MCX cartridges and described protocol, was ≥85%. Chromatographic separation using the low dispersion ACQUITY UPLC I-Class System and BEH Phenyl 1.7 um Column, provided excellent resolution and separation from endogenous matrix interferences. The Xevo TQ-XS tandem quadrupole mass spectrometer, equipped with a novel StepWave ion guide, enabled improved ion sampling in the source and ion transfer efficiency, while use of the UniSpray ionization technique improved sample ionization improving signal-to-noise (S/N) and detection limits. With this method, lower limits of quantification (LLOQ) of 0.2 pg/mL were achieved with a S/N of 12.
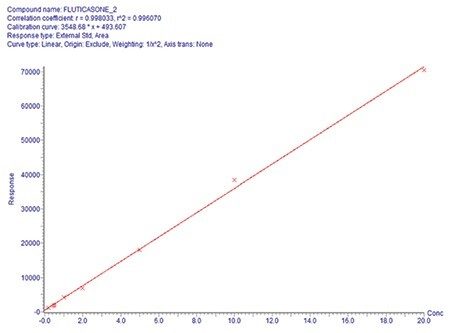
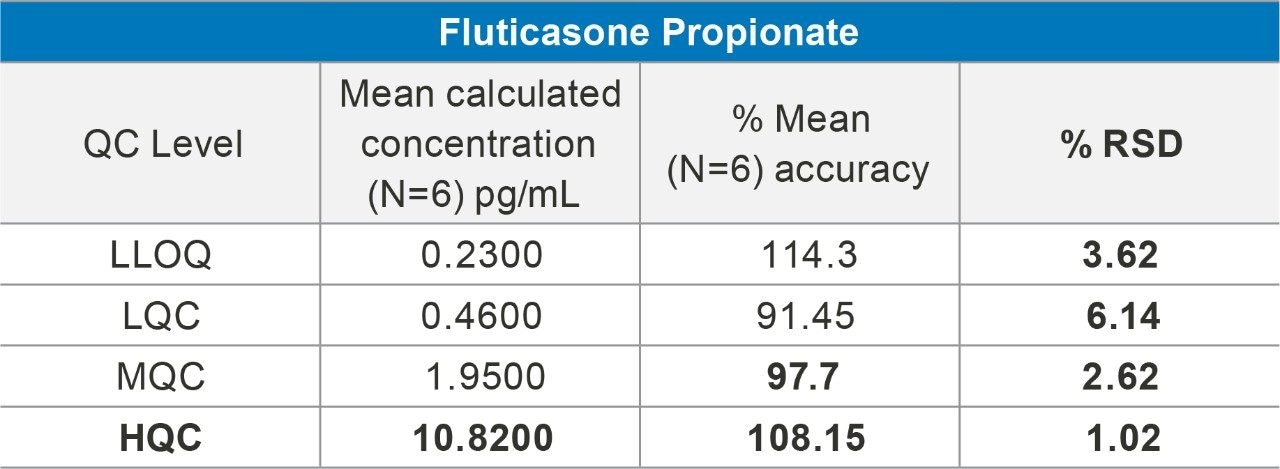
Overall quantification performance was excellent. The method delivered a linear calibration response over the range of 0.2–20 pg/mL (Figure 4) with a correlation coefficient of 0.9960 (Figure 3). At the same time, QC statistics for PA batches easily met recommended bioanalytical method development guidelines 3, with accuracy values ≤15% with excellent single digit precision (≤6%) for all QC levels (Table 1).
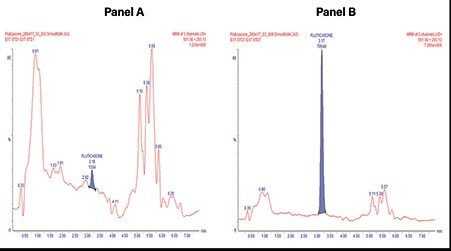
In this work, a simple sample preparation strategy with Oasis MCX SPE combined with UPLC chromatographic separation ACQUITY UPLC I-Class System, and sensitivity MS analysis, using the Xevo TQ-XS Mass Spectrometer was used to successfully quantify fluticasone propionate from plasma. This method achieved sub-pg/mL detection sensitivity, achieving LLOQs of 0.2pg/mL with a linear dynamic range 0.2-20pg/mL. The excellent quantitative performance of the method described herein, reliably measures low levels of fluticasone propionate, demonstrating its suitability and fit-for-purpose in support of drug discovery and research.
720006422, May 2019