
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates superior performance of the ACQUITY UPLC H-Class PLUS System with UV and mass detection for accurate recording of method development activities towards efficient separation, detection, and quantitation of sunscreen actives.
The ACQUITY UPLC H-Class Plus System coupled to Empower 3 CDS Software offers powerful tools to streamline method development activities.
Skin cancer is the most common type of cancer diagnosed in the U.S.; it is estimated one in five Americans will develop skin cancer in their lifetime.1-4 The most serious class of skin cancer is the melanomas, which develop in the melanocyte cells of skin. The World Health Organization (WHO) estimates 65,000 mortalities per year are attributed to melanomas.5 Around 95% of melanomas are caused by exposure to UV radiation.6
Due to the damaging effects of UV light on skin, increasing numbers of cosmetics and personal care products are formulated with chemicals that actively filter out UV radiation. However long term contact with chemical sunscreens may increase the risk of developing a skin allergy to sunlight.7 For this reason, the type and amount of sunscreen agents in formulations have been strictly regulated around the world and effective methods for simultaneously detecting multiple chemical sunscreen agents in formulations are necessary.8 HPLC has been applied extensively to this application.9 However, drawbacks associated with the methods published to date include prohibitive analysis times on HPLC scale columns and/or the use of toxic solvents.
In this technology brief, we demonstrate equivalent performance of Waters ACQUITY UPLC H-Class PLUS System for the separation and detection of a mix of six chemical UV filters by measuring and comparing typical system suitability parameters to the data acquired on an ACQUITY UPLC H-Class System on two different column chemistries.
Analytical standards were purchased from Sigma-Aldrich Ltd (Poole, Dorset, UK). The standards were prepared at concentrations of 25 µg/mL in 60:40 water:MeOH for UPLC analysis. UPLC separation was achieved on the ACQUITY UPLC HSS PFP Column (1.8 µm, 2.1 x 50 mm, p/n: 186005965) and the ACQUITY UPLC BEH C8 Column (1.7 µm, 2.1 x 50 mm, p/n: 186002877) at a flow rate of 0.5 mL/min using a general gradient: 40% B initially to 1 min grading to 95% B at 4.5 min, followed by a 2.5 min hold before returning to 40% B and re-equilibrating for 1 min. The mobile phase consisted of A: water and B: methanol, each with 0.1% formic acid, and the injection volume was 5 µL. The retention times and peak areas were measured by Empower 3 CDS Software and were the average of five replicate injections.
Six sunscreen actives were analyzed on both column chemistries using the same mobile phase, gradient, and injection volume by both the ACQUITY UPLC H-Class and the ACQUITY UPLC H-Class PLUS systems. Figure 1 shows chromatograms for the six compounds on both systems using the ACQUITY UPLC BEH C8 Columns. Figure 2 shows the same six analytes separated using ACQUITY UPLC HSS PFP Column across both systems. The retention times and peak areas between the two systems, shown in Figure 3, were comparable for all of the compounds tested. Retention time reproducibility as illustrated by %RSD was less than 0.2% and 0.04% for the ACQUITY UPLC H-Class and ACQUITY UPLC H-Class PLUS systems respectively. These data demonstrate the reproducibility across the ACQUITY UPLC H-Class and H-Class PLUS systems and show that methods previously validated on the ACQUITY UPLC H-Class systems should not need revalidating for performing analyses on the ACQUITY UPLC H-Class PLUS System.

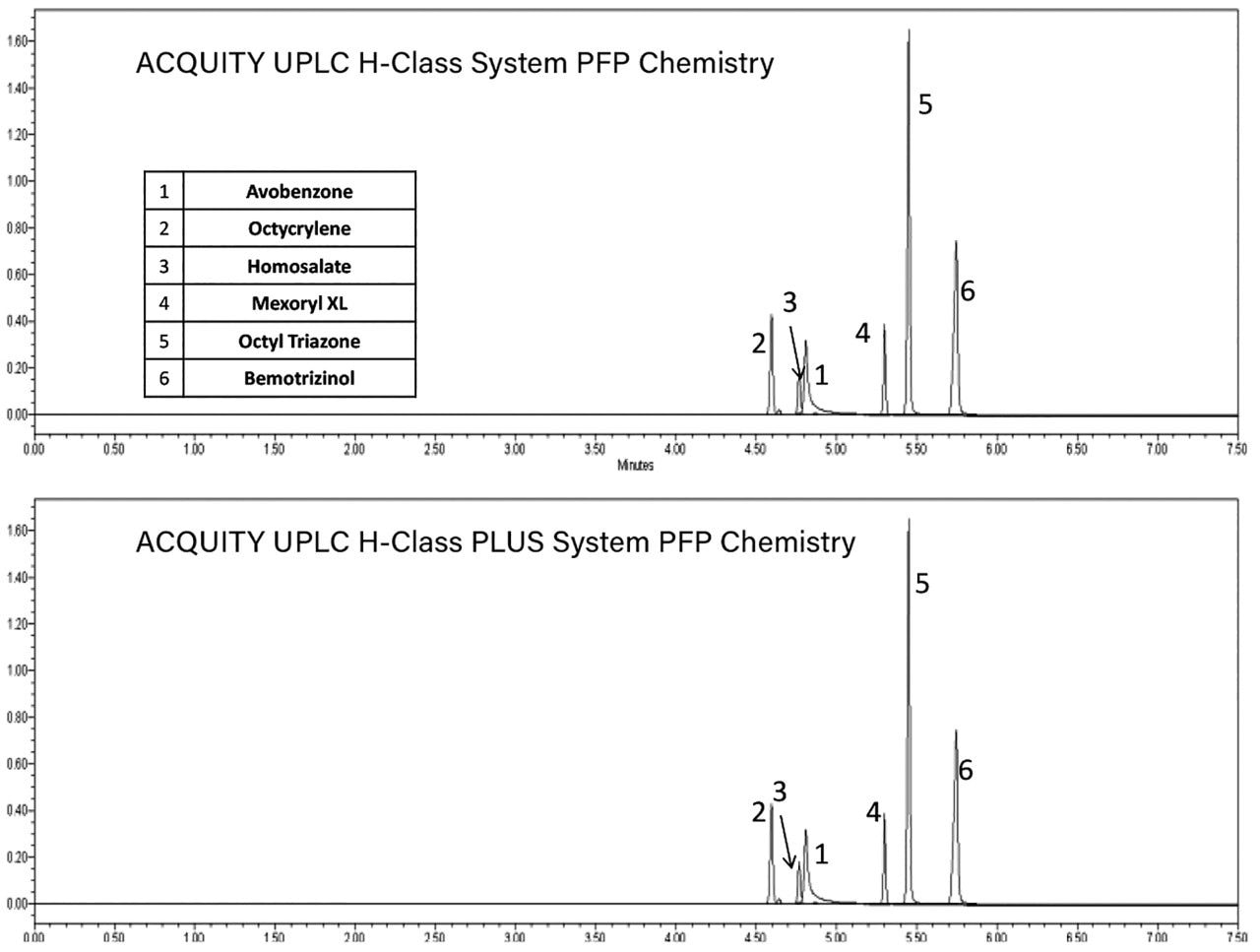
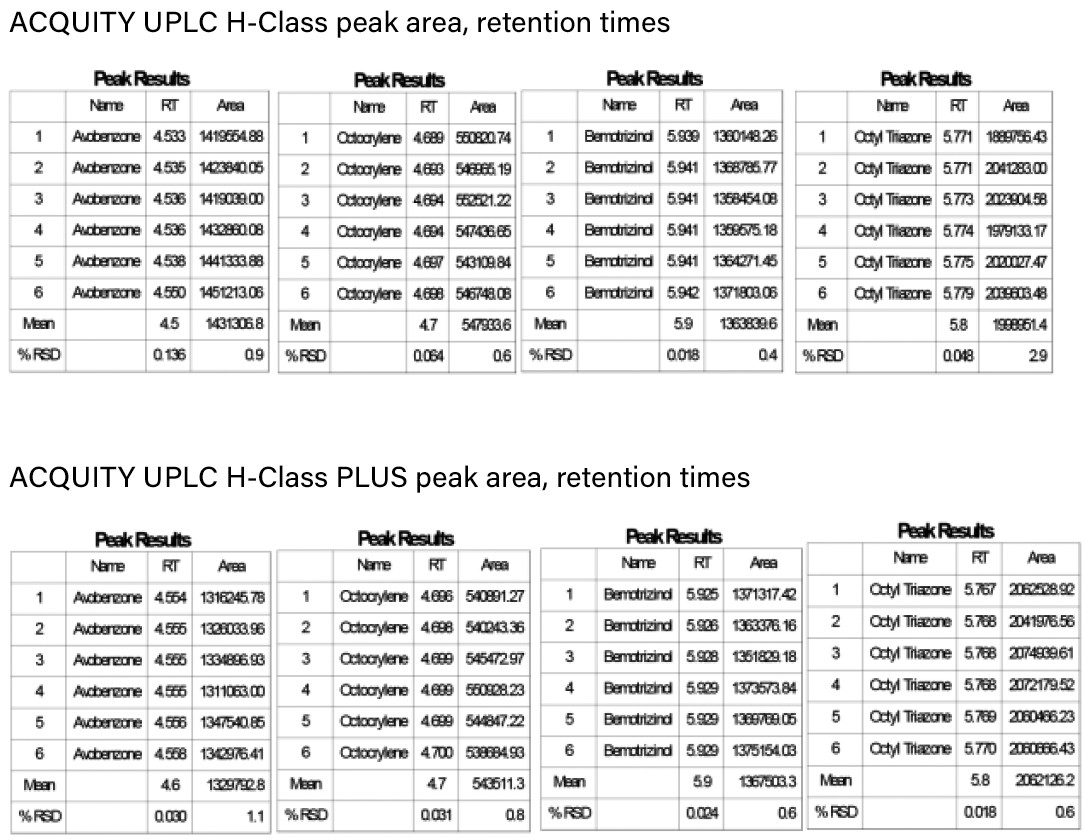
The ACQUITY UPLC H-Class PLUS System is a robust and reliable analytical technology for the accurate analysis of sunscreen actives in cosmetic formulations. The enhanced reproducibility of the system will aid in the identification of batch to batch variations in QC monitoring of sunscreen manufacture.
720006283, May 2018