This is an Application Brief and does not contain a detailed Experimental section.
Protein quantification workflows are often complex and laborious, and extensive sample clean-up to achieve specificity and sensitivity for accurate quantification from complex biological matrices is required. While affinity purification at the protein level can significantly increase the ultimate sensitivity of the assay, the cost and time necessary are not realistic for discovery studies. Identification and implementation of a simpler pre-fractionation, such as protein precipitation, can improve sensitivity, while a standardized, kit-based approach minimizes method development. Both provide a common generic option for inexperienced analysts to achieve sensitive, accurate and robust quantification of proteins.
Demonstrates the compatibility of ProteinWorks Kits with pellet digestion and to show improved sensitivity/selectivity for the monoclonal antibody (mAb) trastuzumab (Herceptin) by combining by pellet digestion and ProteinWorks eXpress Direct Digest Kits.
Due to the similarity of therapeutic proteins with each other and other plasma/serum proteins, there are often significant matrix and isobaric interferences which can severely limit selectivity and sensitivity of a quantitative LC-MS assay for antibody and other proteinbased drugs. One of the major culprits in this respect is serum albumin. Present at 35–50 mg/mL, albumin not only interferes significantly and causes ion suppression chromatographically, but its presence can also reduce digestion efficiency, and at the very least, increases the amount of enzyme required, adding significant cost to the assay. For this reason, additional clean-up strategies (e.g., protein precipitation, immuno-capture with Protein A/G or specific capture reagents, solid phase extraction, and molecular weight cut-off filters) are often employed to reduce sample complexity and to impart additional specificity and sensitivity. Of these techniques, protein precipitation (PPT) with organic solvents prior to protein digestion is the most attractive. PPT results in an aqueous/organic layer containing small molecules, phospholipids, salts, and some proteins. The pellet typically contains larger precipitated proteins. Depending on the choice of organic and the ratio of organic to plasma, it is possible to precipitate proteins such as antibodies, while maintaining solubility of the majority of albumins in the liquid layer so that they may be removed. This has the advantage of being a simple, inexpensive, and fast way to enrich antibodies and other large proteins while removing the majority of interfering albumins, detergents, small proteins, phospholipids and other endogenous components of biological matrices. Ultimately, incorporating this option into a generic, yet standardized LC-MS approach for protein bioanalysis enables novice scientists to more successfully support discovery studies.
Preliminary experiments were conducted using a series of PPT conditions such as varying the starting plasma volume, ratio of organic to plasma, nature of organic, and time and rate of centrifugation. To measure performance, raw area counts from tryptic peptides of both human serum albumin (HSA) and trastuzumab were monitored. Conditions which produced the greatest removal in albumin (as represented by a reduction in albumin peptide area counts) while maintaining or improving trastuzumab sensitivity/recovery (as represented by increased trastuzumab peptide area counts) were progressed for further study.
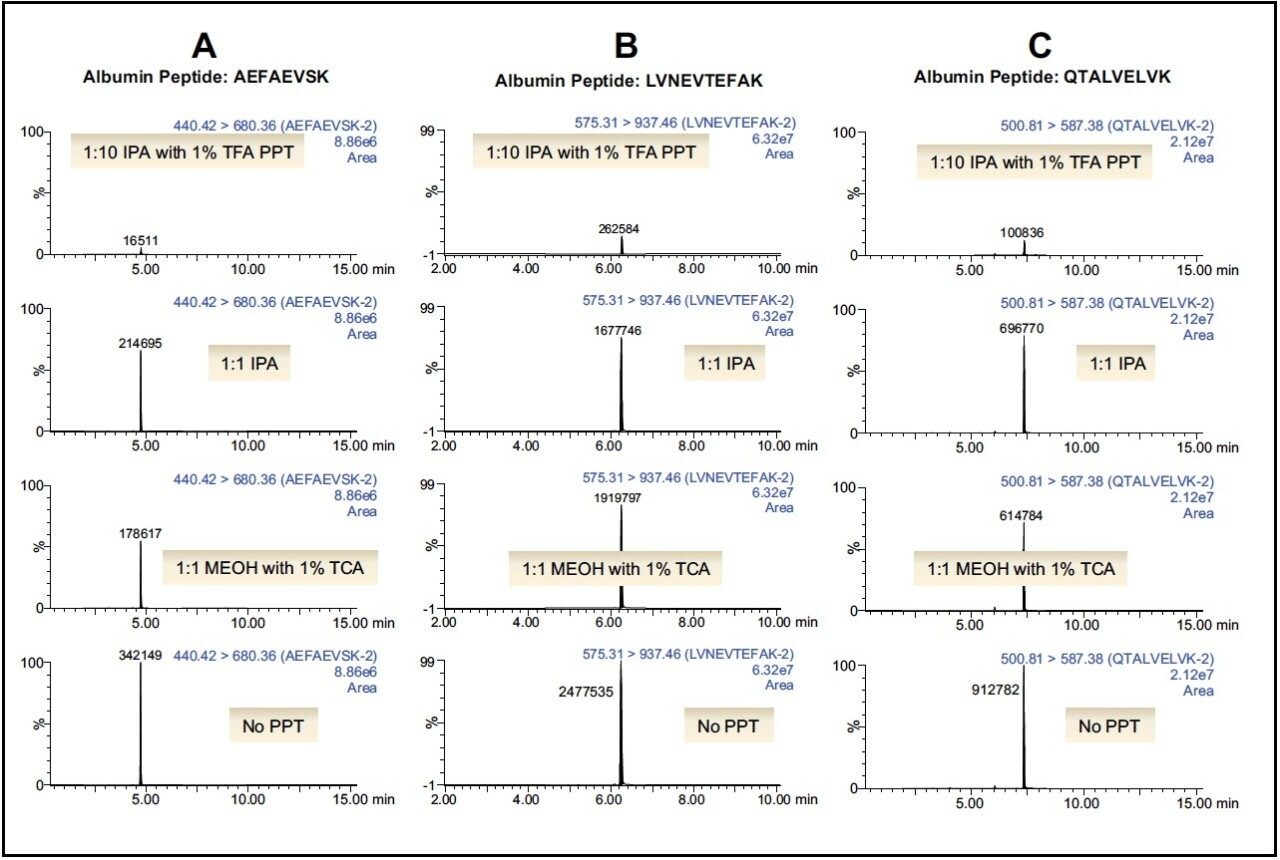
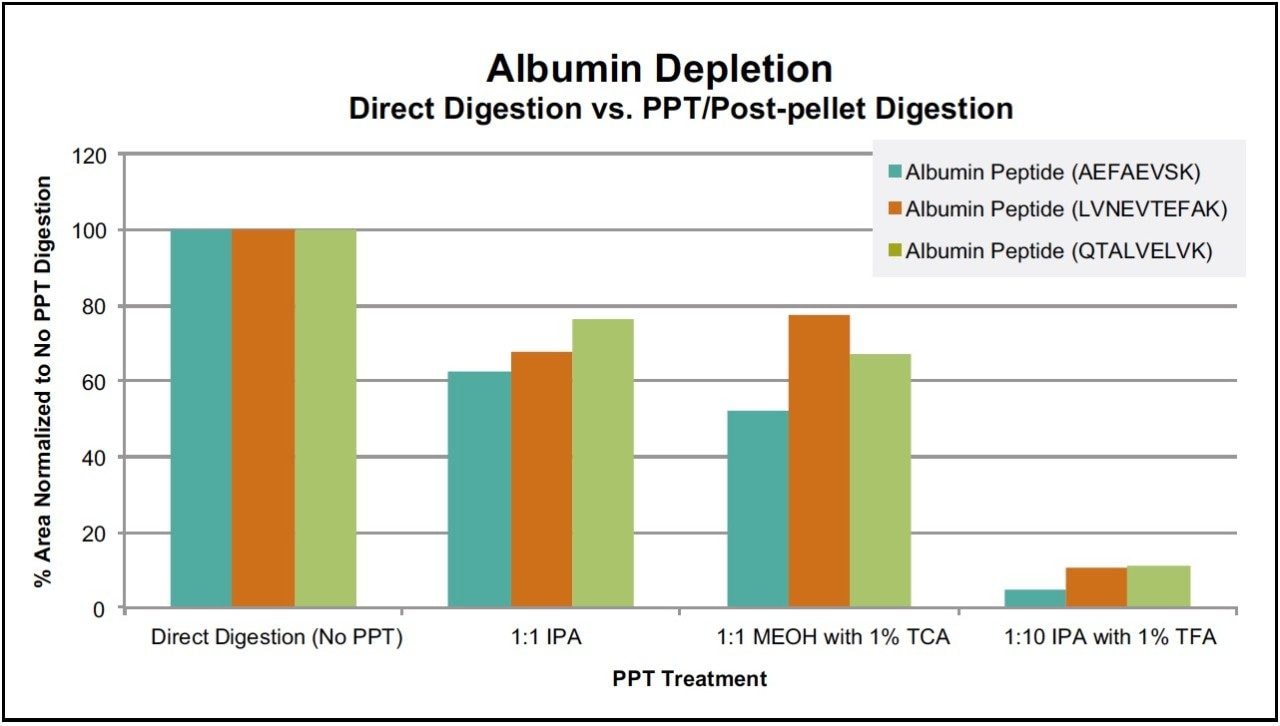
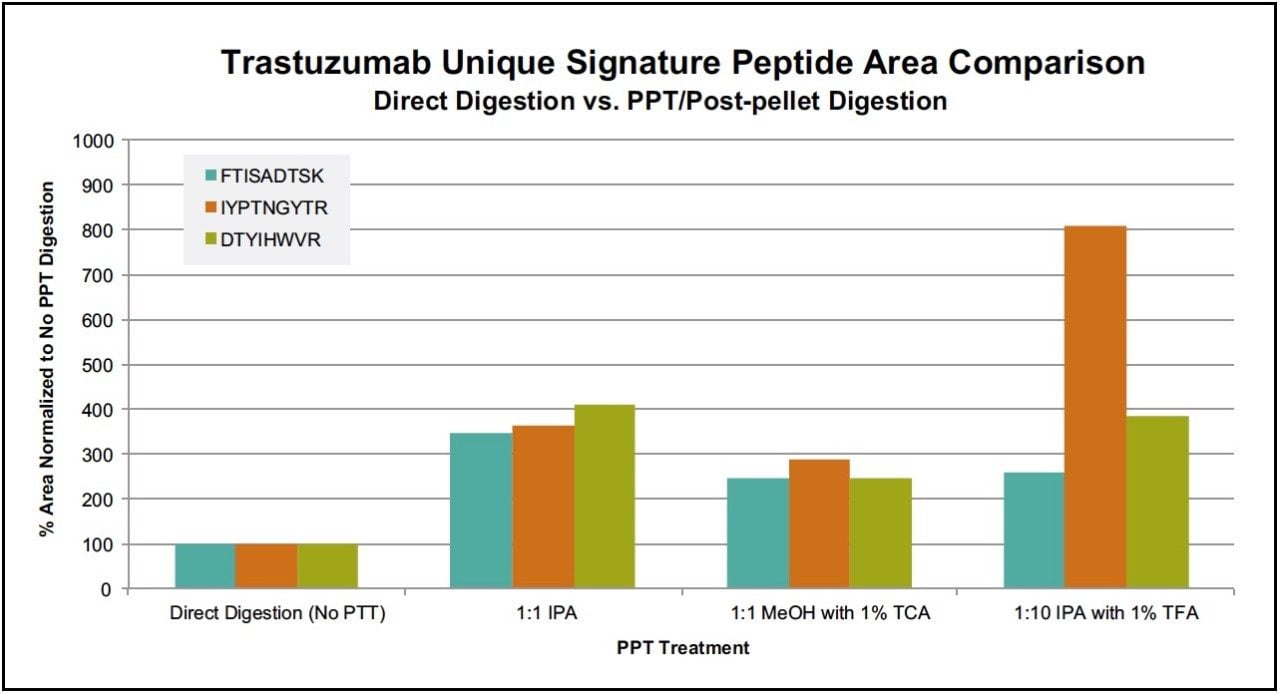
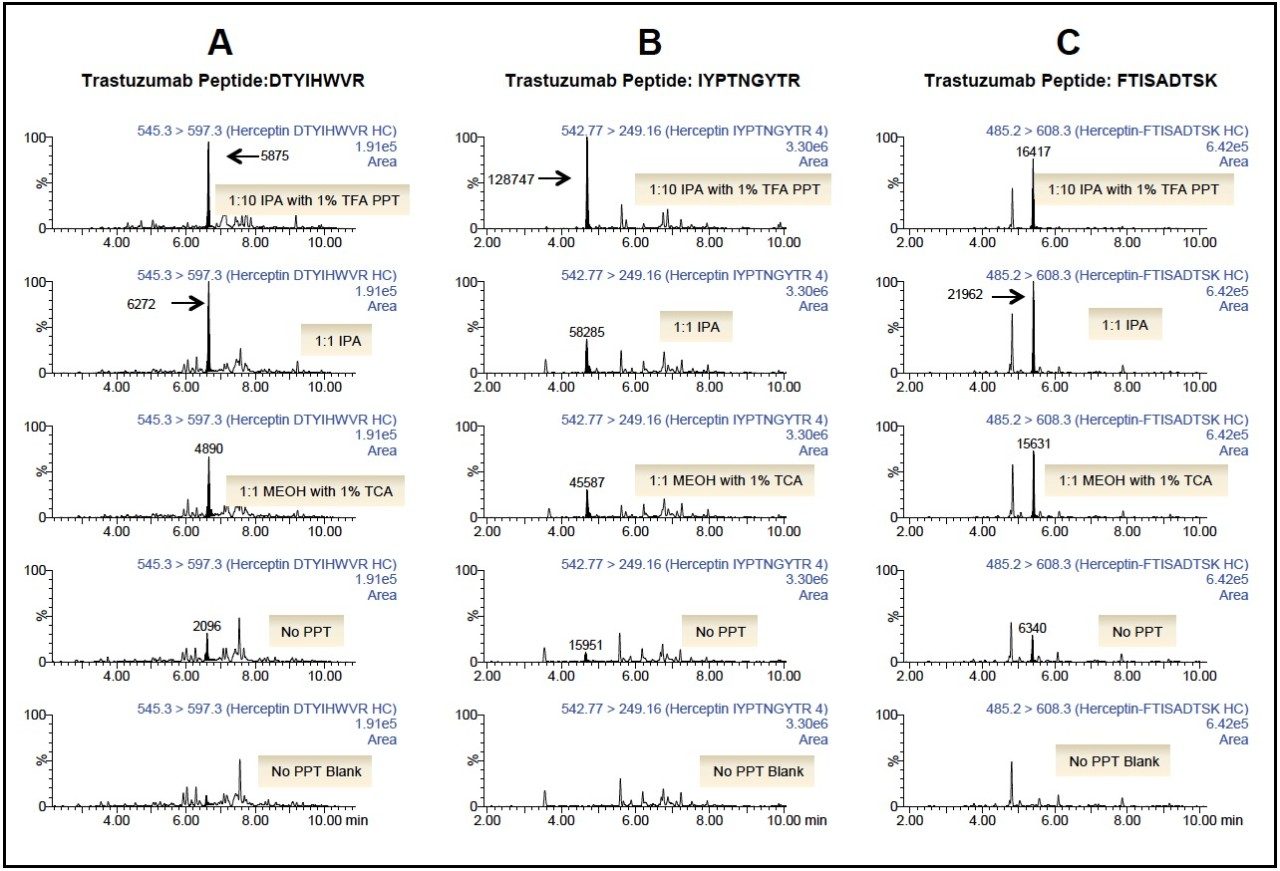
Following PPT of plasma samples (15 μL), pellets were re-suspended with buffer and subsequently digested using the ProteinWorks eXpress Direct Digest kit and protocol. Using a targeted UPLC-MS/MS method for the representative tryptic peptides (mAb and albumin), digested plasma samples with and without the PPT pre-treatment were analyzed. Of the various plasma PPT pre-treatments, three PPT conditions (1:1 isopropanol, 1:1 methanol containing 1% trichloroacetic acid, and 1:10 isopropanol containing 1% trifluoroacetic acid) were chosen based on sensitivity increases in the mAb tryptic peptides (area count) and reduction of several human serum albumin peptides (area count) when compared to non-PPT treated plasma samples. The reduction of 3 particular albumin peptides (AEFAEVSK, LVNEVTEFAK, and QTALVELVK) with the various PPT treatments is demonstrated in Figure 1, panels A–C. While all 3 treatments provided some degree of albumin removal, PPT with isopropanol (IPA) with 1% trifluoroacetic acid (TFA), at a ratio of 1:10, provided the most effective removal of the albumin peptides (>90%), as shown in Figure 2. Area counts for all 3 unique tryptic peptides from trastuzumab increased significantly using any of the top three PPT conditions (Figure 3). In fact, for the three best PPT pre-treatments, peptide area counts increased 2–8x on average, as is demonstrated in Figure 4.
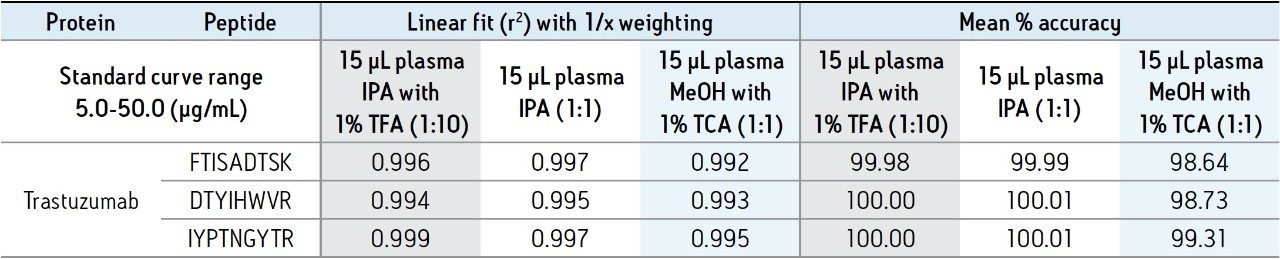
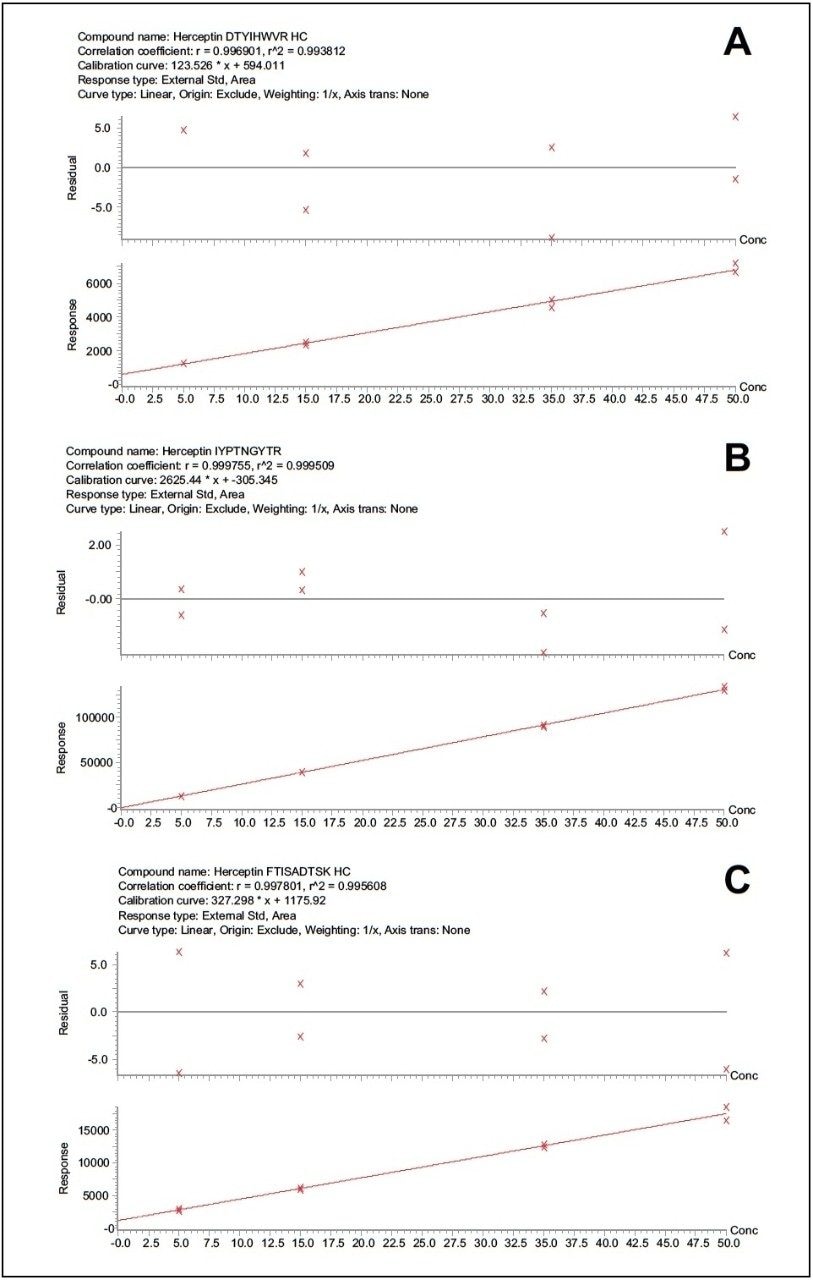
Standard curves were then prepared in plasma. 15 μL aliquots were subjected to the different PPT conditions, followed by the generic ProteinWorks eXpress Direct Digest Kit and Protocol. Three unique trastuzumab peptides were assessed for linearity and accuracy as an initial gauge of performance. For all PPT treatments, and using the ProteinWorks kits for digestion, standard curves for all peptides were linear with R2 ≥0.99, using 1/x weighting. Mean % accuracies of the standard curve points were >99% (Table 1). Representative calibration curves for the three trastuzumab tryptic peptides (DTYIHWVR, IYPTNGYTR, and FTISADTSK) prepared using PPT pre-treatment (1:10 IPA containing 1% TFA) of the plasma samples and pellet digestion with ProteinWorks eXpress Direct Digest Kits, are illustrated in Figure 5, panels A–C.
We describe here a robust and reproducible pellet digestion and LC-MS/MS methodology to quantify the mAb trastuzumab. A simple PPT clean-up and post-pellet digestion using the ProteinWorks eXpress Direct Digest Kit yields accurate, precise and robust LC-MS quantification of trastuzumab via the surrogate peptide approach. Area counts for trastuzumab peptides increased 2–8x using the above strategy, significantly improving sensitivity and specificity, whilst achieving accurate and reproducible quantitative results.
720005574, January 2016