The USP compendial method for the assay of Zidovudine requires a 5 μm, 4.0 x 250 mm L1 column. This method will be transferred from the original column to both a CORTECS C18, 2.7 μm, 4.6 x 150 mm Column and a CORTECS C18, 2.7 μm, 4.6 x 100 mm Column to demonstrate two possible transfers. Both of these transfers will be allowable under USP General Chapter <621>, starting in August of 2014.
In pharmaceutical manufacturing, lengthy USP compendial methods can drastically slow down sample analysis, limiting productivity. Furthermore, these methods can carry significant cost-per-analysis due to mobile phase consumption. Transferring these compendial methods, typically performed on large dimension 5 μm columns, to new CORTECS 2.7 μm Solid-Core Particle Columns can drastically reduce analysis time and solvent usage.
Zidovudine is an antiretroviral drug used in the treatment of HIV and AIDS. The USP compendial method for the assay of Zidovudine requires a 5 μm, 4.0 x 250 mm L1 column.1 This method will be transferred from the original column to both a CORTECS C18, 2.7 μm, 4.6 x 150 mm Column and a CORTECS C18, 2.7 μm, 4.6 x 100 mm Column to demonstrate two possible transfers. Both of these transfers will be allowable under USP General Chapter <621>, starting in August of 2014.2 The changes to the chapter allow for alterations in the method based on the column length to particle size ratio (L/dp) and the number of theoretical plates (N). By using the two CORTECS 2.7 μm Columns, each of these possible transfers will be examined.
During the transfer study, instrument Performance Qualification (PQ) will be done using the Neutrals Quality Control Reference Material (QCRM). By using the QCRM, which is intended to evaluate system performance, an analyst can monitor the system, and potentially detect system problems earlier. The QCRM was used to bracket sample analysis to ensure that the data collected was done so while the system was operating properly.
|
System: |
Alliance HPLC |
|
Columns: |
Fully-porous C18, 5 μm, 4.6 x 250 mm; CORTECS C18, 2.7 μm, 4.6 x 150 mm (p/n 186007378); CORTECS C18, 2.7 μm, 4.6 x 100 mm (p/n 186007377) |
|
Mobile phase: |
80:20 water:methanol |
|
Separation mode: |
Isocratic |
|
Flow rates: |
1.0 mL/min (4.6 x 250 mm column); 0.9 mL/min (4.6 x 150 mm column); 1.5 mL/min (4.6 x 100 mm column) |
|
Column temp.: |
30 °C |
|
Detection (UV): |
265 nm |
|
Injection volume: |
10.0 μL (4.6 x 250 mm column); 6.0 μL (4.6 x 150 mm column); 5.3 μL (4.6 x 100 mm column) |
|
Vial: |
LCMS Certified Max Recovery Vial (p/n 600000749CV) |
|
QCRM: |
Neutrals QCRM (p/n 186006360) |
|
Data management: |
Empower 3 CDS |
Zidovudine and its related compounds B and C reference standards were purchased from the USP. A sample containing 1.0 mg/mL Zidovudine, 1.0 μg/mL related compound B, and 2.0 μg/mL related compound C was created in methanol and placed into a Waters LCMS Certified Max Recovery Vial injection.
As part of the method transfer, a reference material was used to bracket sample analysis. This acts as a check on the system to confirm the accuracy of the data collected. In this case, the QCRM was used. The Neutrals QCRM was used to bracket the analysis of samples to ensure that the data collected was accurate and that the system, including the column, was functioning properly over the course of the analyses. The Neutrals QCRM consists of three neutral compounds, which are compatible with a wide variety of mobile phases and column chemistries, making it suitable for all types of reversed phase analysis. Figure 1 shows injections from before and after sample analysis on the three test columns.
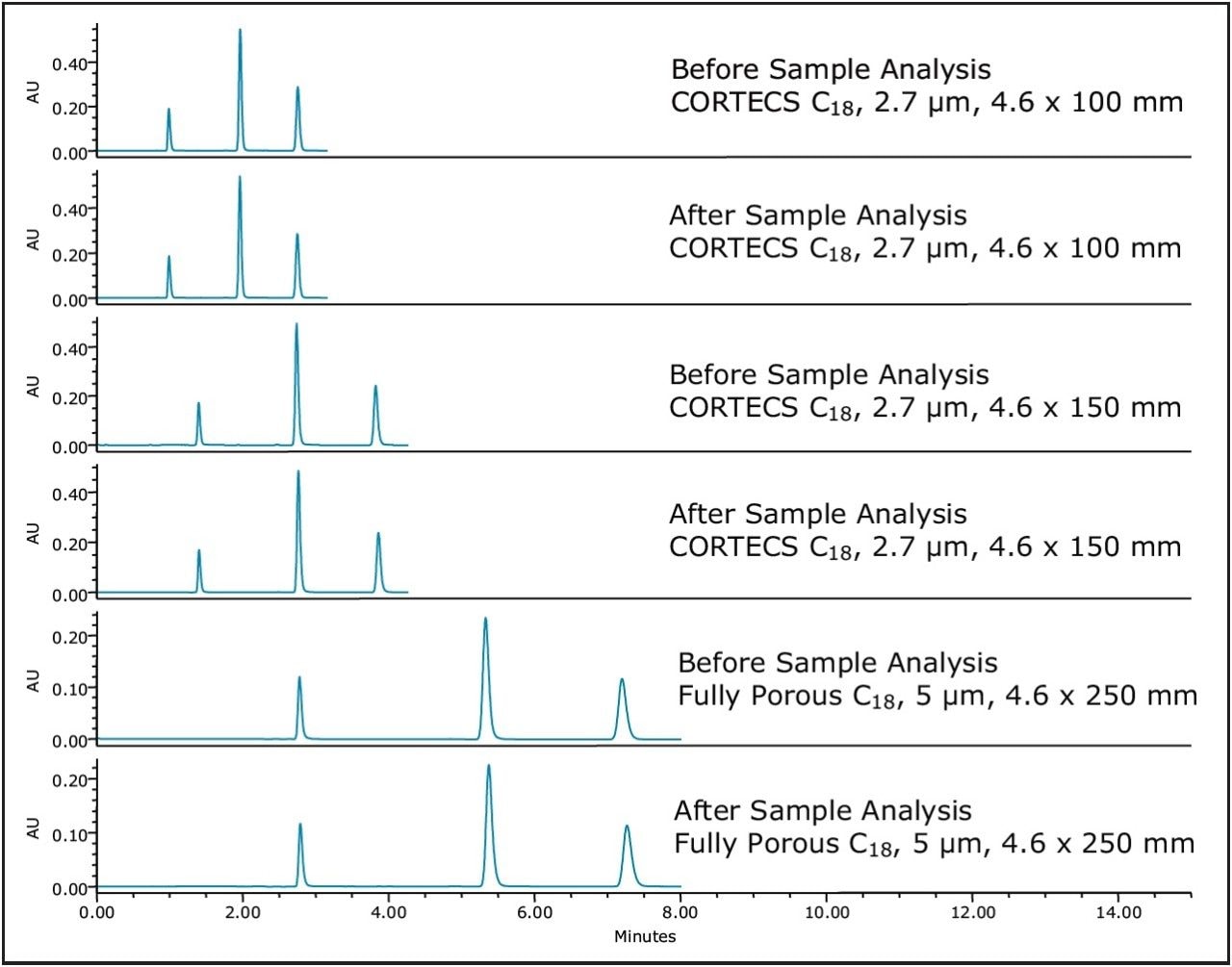
As Figure 1 shows, the bracketed injections of the Neutrals QCRM show reproducible results. This confirms that the data collected during the method transfer on each test column is accurate. Having a procedure which checks system performance can help to detect system problems earlier, reducing potential system downtime.3
USP General Chapter <621> outlines changes that can be made to a monograph without the need for revalidation. According to these guidelines, which become official in August 2014, any change can be made to the length and particle size of a column as long as the ratio of column length to particle size (L/dp) is within -25% and +50% of the original column dimensions for isocratic methods. In the case of Zidovudine, the original column L/dp ratio is 50,000. By using a CORTECS C18, 2.7 μm, 4.6 x 150 mm Column (L/dp= 55,555) the guidelines for allowable changes have been met. The CORTECS Column has an 11% higher L/dp than the original column. L/dp is a measure of a column’s maximum resolving power. The higher the L/dp ratio, the greater resolution the column can potentially generate. Figure 2 shows the separation of Zidovudine and its related compounds on both the original 5 μm column, and a 150 mm CORTECS 2.7 μm Column.
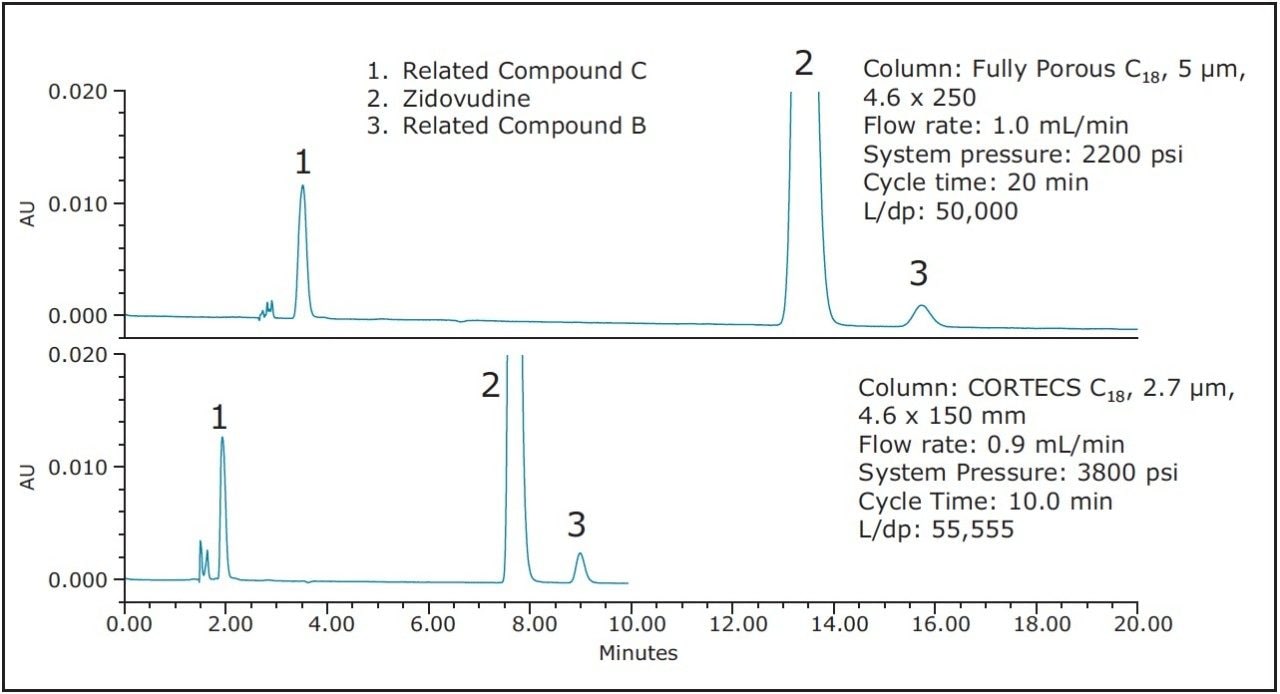
As Figure 2 shows, a 50% reduction in cycle time and solvent usage can be achieved by using a CORTECS C18, 2.7 μm, 4.6 x 150 mm Column. By choosing to maintain L/dp an analyst can be reasonably certain that the separation will meet the monograph criteria. In the case of the Zidovudine assay, the monograph outlines three system suitability criteria that must be met in order for the assay to pass. Table 1 outlines the criteria and the required results, as well as the results obtained from 5 replicate injections of the sample on each column. As the data shows, the CORTECS 4.6 x 150 mm Column meets all the required system suitability criteria. By using this column, cycle times and solvent usage are reduced, potentially increasing laboratory productivity while reducing cost, all without the need for method revalidation.
Transfer of the USP monograph based upon number of theoretical plates (N) General Chapter <621> has an additional avenue for method transfer of isocratic methods. Starting in August 2014, a column can be used for transfer if the number of theoretical plates (N) is within -25% and +50% of the original column. The number of theoretical plates is a measure of column efficiency, and can be influenced by the separation parameters. In order to calculate N the peak width at baseline and retention time of a peak must be recorded. Equation 1 is used to calculate N, where tR is retention time of the peak, and W is the width at baseline for the peak.
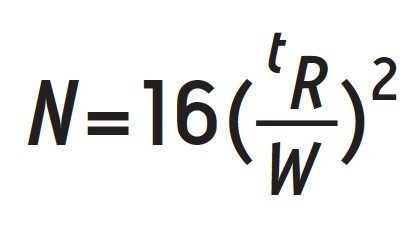
Once N has been calculated for a particular column running a separation, then a new column can be used as long as the number of theoretical plates for the new column is within the guidelines outlined by general chapter <621>. In this application the average N value for the original 5 μm, 4.6 x 250 mm column using the latest eluting peak was 2957 plates. In order to stay within the USP guidelines, the column that is used for transfer must have between 2217–4435 plates for the latest eluting peak. The transfer was performed using a CORTECS C18, 2.7 μm, 4.6 x 100 mm Column. Figure 3 shows the separation on the two columns.
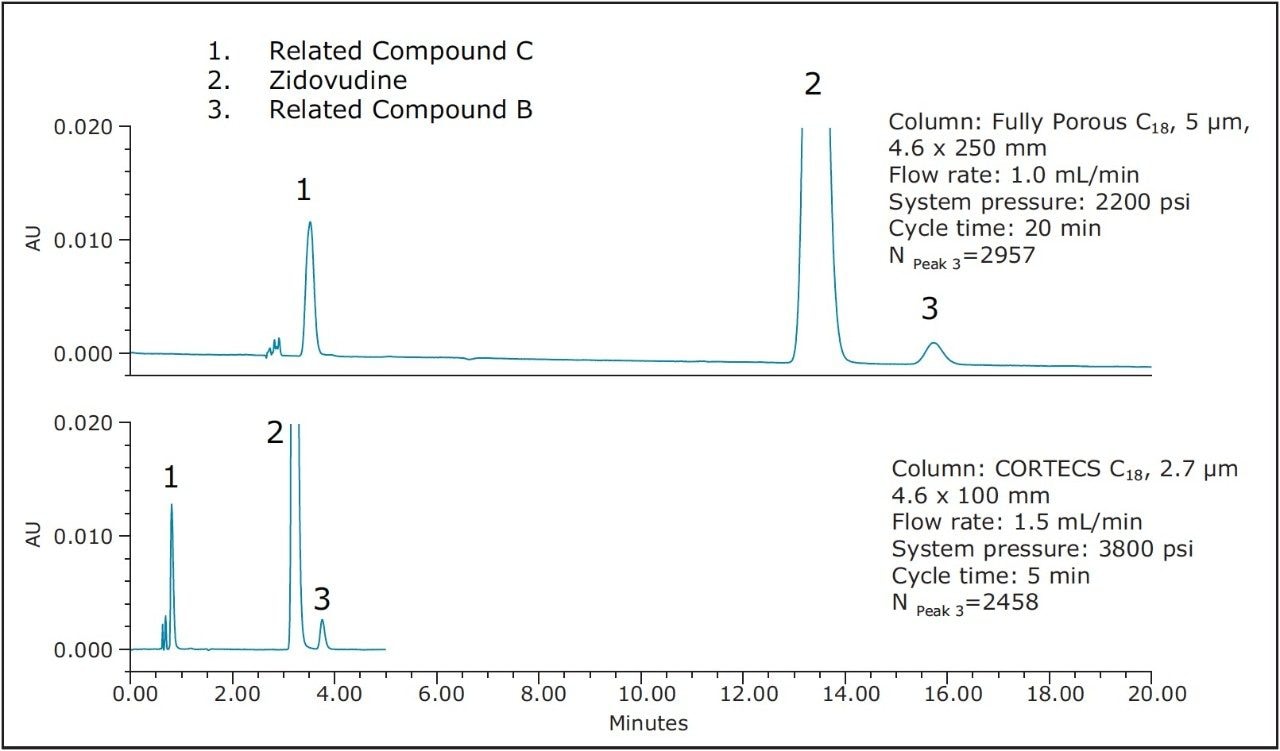
As Figure 3 shows, the separation using a CORTECS 4.6 x 100 mm Column is allowable under the guidelines. With an N value of 2458 for the last peak the separation is within the -25% guideline. By using this column cycle times are reduced by 75%, greatly increasing sample throughput. Furthermore, solvent usage is reduced by 63% saving a significant amount of solvent per analysis. Table 1 also shows that the system suitability criteria for the assay on the CORTECS 4.6 x 100 mm Column pass.
USP compendial methods are traditionally performed on 5 μm columns, and take a considerable amount of time and solvent to perform an analysis. Starting in August 2014, the official USP general chapter <621> will outline two ways to transfer isocratic methods to smaller particle columns. The first is by maintaining the length to particle size ratio (L/dp). The second is by maintaining the number of theoretical plates (N). The isocratic separation of Zidovudine was transferred to two separate CORTECS 2.7 μm columns using both methods of transfer to show the versatility of the new solid-core columns. The separation can be performed up to 4 times faster using CORTECS 2.7 μm Columns, with decreased solvent usage. Additionally, by following the USP General Chapter <621> guidelines, there is no need to re-validate the method, allowing for a quick transfer between columns. These benefits can lead to increased laboratory productivity, and reduced cost per analysis.
720005157, October 2014