
The analysis of animal feedingstuffs including silage represents a major technical challenge due to the complexity and in-homogeneity of these matrices. Although permitted limits for mycotoxins are set at relatively high (μg kg-1) concentrations in the EU, toxic effects such as immunotoxicity and feed uptake problems in certain species (poultry and porcine) are often observed at sub μg kg-1 concentrations. For this reason there is often a requirement to achieve low detection limits in feedingstuffs. There is also a potential for co-contamination due to pre- and post- harvest infestation resulting in the occurrence of tricothecenes, beauvercin and enniatins, fumonisins, ochratoxin, T2, HT-2, and alternaria toxins for example within a single feed sample. In this application note, we report the development of a quantitative method for the determination of 33 relevant mycotoxins in a variety of animal feed and silage extracts. The ACQUITY UPLC I-Class System coupled to a Xevo TQ-S was used for rapid, high quality, and ultra-sensitive analysis of multiple mycotoxins in feed extract. Our goal was to investigate the effect of matrix dilution and enhanced instrument sensitivity to overcome common analytical challenges such as ion suppression and to reduce the effects of matrix variability.
There are now over 400 recognized mycotoxins that may be found in animal feedings materials and it has been reported that as much as 25% of the world’s cereal grains may be contaminated with mycotoxins.1
The analysis of animal feedingstuffs including silage represents a major technical challenge due to the complexity and in-homogeneity of these matrices. Although permitted limits for mycotoxins are set at relatively high (µg kg-1) concentrations in the EU,2,3 toxic effects such as immunotoxicity and feed uptake problems in certain species (poultry and porcine) are often observed at sub μg kg-1 concentrations.4 For this reason there is often a requirement to achieve low detection limits in feedingstuffs. There is also a potential for co-contamination due to pre- and post- harvest infestation resulting in the occurrence of tricothecenes, beauvercin and enniatins, fumonisins, ochratoxin, T2, HT-2, and alternaria toxins for example within a single feed sample.5
In this application note, we report the development of a quantitative method for the determination of 33 relevant mycotoxins in a variety of animal feed and silage extracts. A Waters ACQUITY UPLC I-Class System coupled to a Xevo TQ-S was used for rapid, high quality, and ultra-sensitive analysis of multiple mycotoxins in feed extract. Our goal was to investigate the effect of matrix dilution and enhanced instrument sensitivity to overcome common analytical challenges such as ion suppression and to reduce the effects of matrix variability.
The extracts of different animal feedingstuffs and silage were kindly provided by RIKILT, The Netherlands and Ghent University for the purposes of this study. A generic and simplified sample extraction protocol based on 84:16 (v/v) acetonitrile: acidified water for the recovery of mycotoxins from the variety of feedingstuffs and silage was used.6 Briefly, the feed samples were mechanically homogenized in the presence of the extraction solvent followed by a centrifugation step. An aliquot of the supernatant was removed and placed in autosampler vial for subsequent LC-MS/MS analysis.

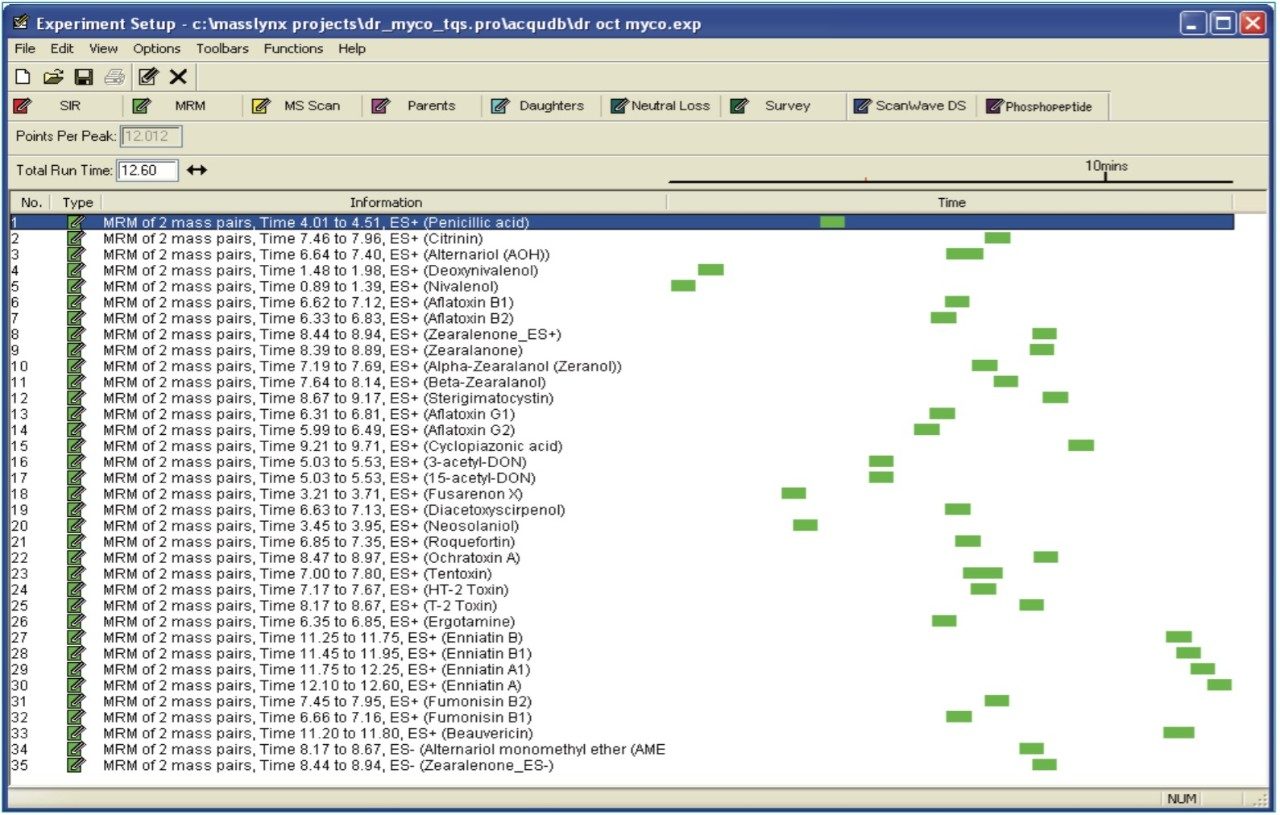
Figure 1 shows the MRM transitions and automated time window scheduling functionality to obtain a minimum of 12 points across each chromatographic peak generated by Quanpedia. This method is available in the Quanpedia database.
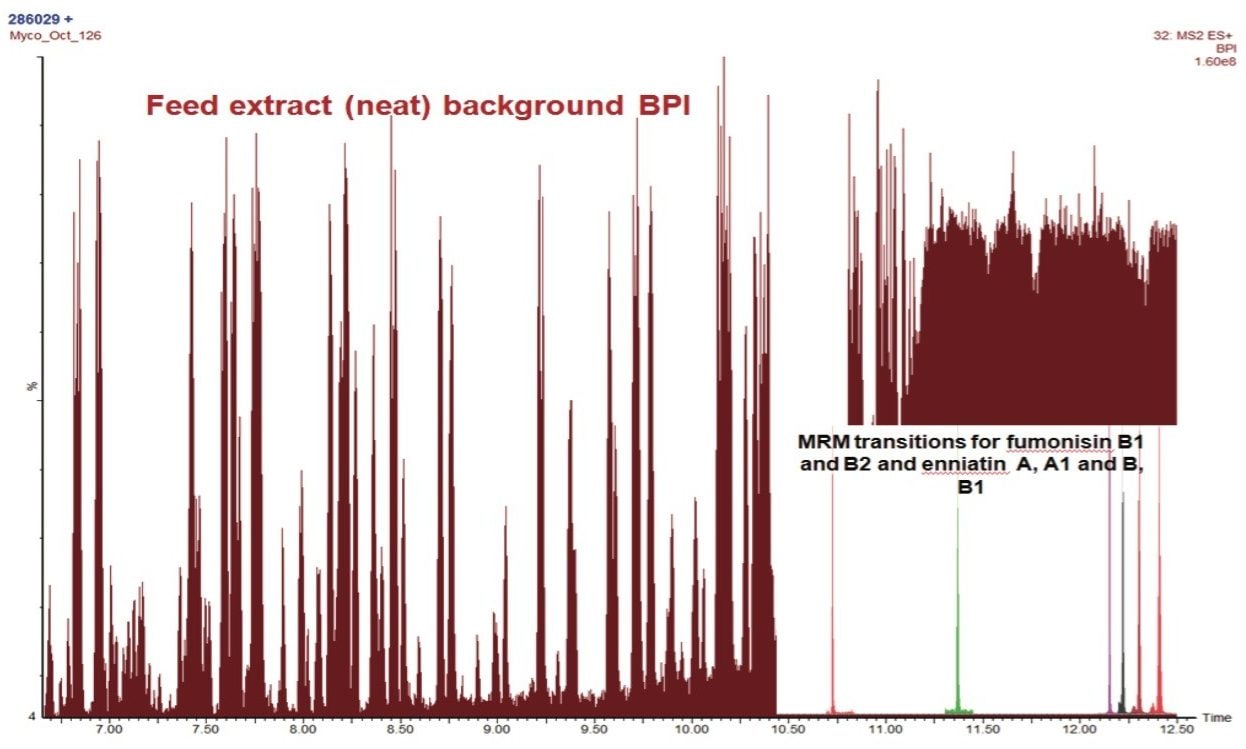
The feed and silage samples were analyzed as neat and diluted extracts and quantified against either solvent or matrix matched standards (as available). As anticipated, the matrix interference profile was found to be highly complex and variable between samples as determined via the RADAR functionality. Figure 2 shows the LC-MS/MS spectra obtained for a neat extract of a porcine feed. The Base Peak Intensity (BPI) spectrum obtained in full scan mode and the simultaneously acquired MRM transitions for five mycotoxins identified in the sample (displayed in the Figure inset) were found to elute in a region of high matrix background. The presence of high concentrations of matrix background can result in variable ion suppression effects for the analytes of interest and can affect the overall analytical performance. For this reason, matrix matched calibrants, standard addition, and isotopically labelled internal standards are approaches typically used for the quantitative analysis of mycotoxins in complex matrices to overcome the matrix effects and improve the quantitative accuracy and precision.
In this study, we investigated the use of a simple dilution step coupled to the enhanced sensitivity of the Xevo TQ-S to reduce the matrix contribution and improve measurement repeatability and accuracy between different sample types. Table 3 shows the repeatability data obtained for six different extracts of feed spiked with six mycotoxins diluted 1:10 prior to injection. The intra-day method precision (expressed as %RSD) was found to be good at less than 23% for all the spiked mycotoxins.
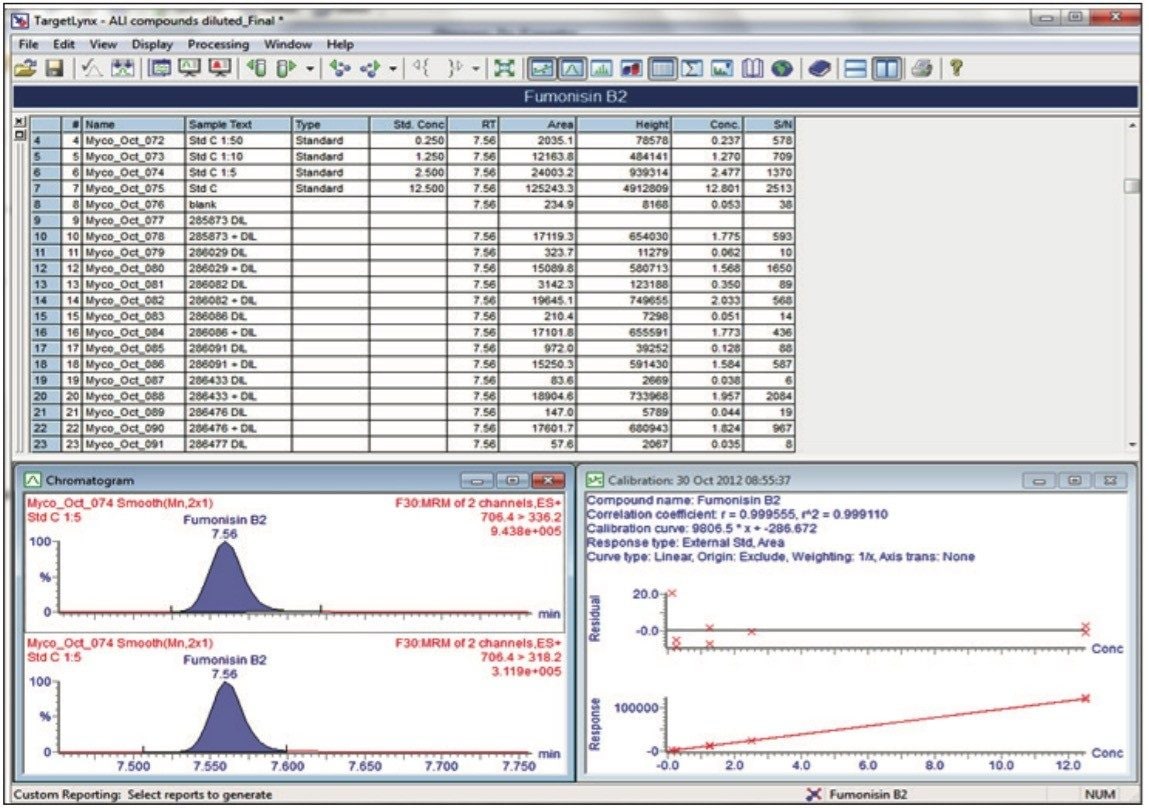
The same dilution factor (1:10) was also applied to a wider selection of feedingstuffs (12) and silage (10) sample extracts and analyzed according to the optimized Xevo TQ-S conditions monitoring for 33 mycotoxins and quantified against an appropriate calibration series. Figure 3 shows the TargetLynx report generated showing the linearity and sensitivity of the method and calculated concentrations for the unknown samples.
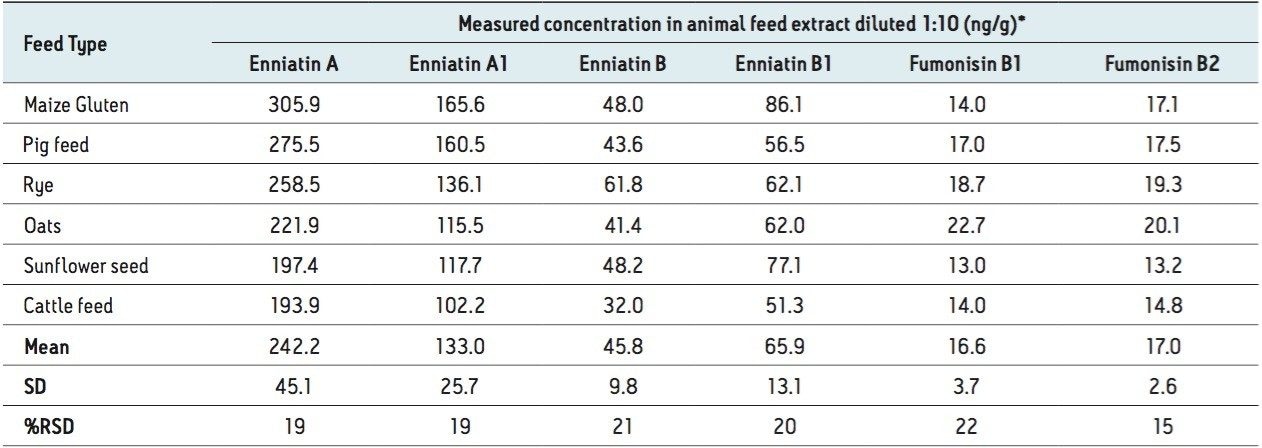
Table 3. The repeatability data generated for a selection of six mycotoxins spiked into to a variety of different animal feeds (n=6), the extracts were diluted 1:10 prior to analysis on the Xevo TQ-S.
*Mycotoxins spiked into feed samples prior to extraction
Tables 4 and 5 show the measured concentrations of the mycotoxins identified (monitoring two MRM transitions) in naturally contaminated feeds and silage samples, respectively along with the calculated LODs (S:N of ≥1:3). The LOD values were found to be in the ppt to low ppb range in both solvent and matrix for all the mycotoxins identified. The naturally contaminated feed samples were found to contain multiple mycotoxins ranging from 3 to 12 of the 33 potential mycotoxins monitored for and estimated to be present at concentrations equivalent sub-1 to circa 300 μg kg-1. The method was considered to be suitable for use as a highly sensitive presence/absence screen as the contaminant concentrations were determined against a solvent standard calibration series, therefore matrix effects may still affect the quantitative performance.
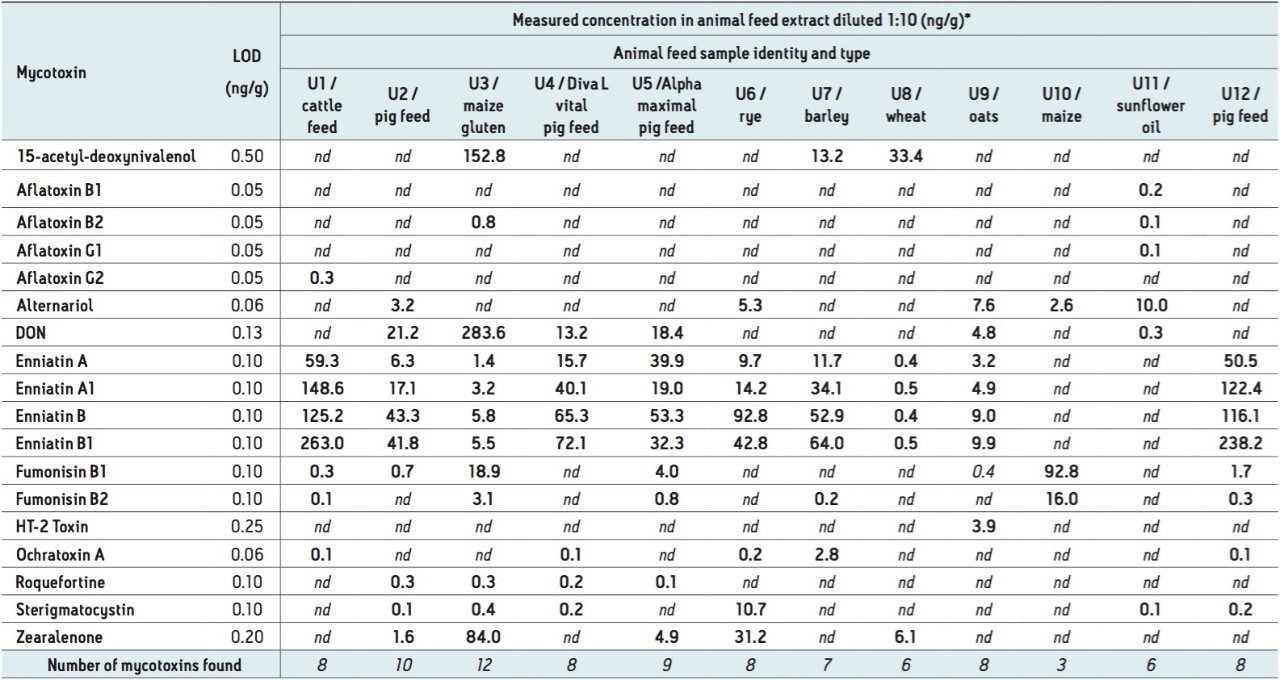
Table 4. The measured concentrations for a range of mycotoxins identified using two MRM transitions in 12 different samples of animal feedingstuffs diluted 1:10 prior to analysis on the Xevo TQ-S.
*Concentration determined against a solvent calibration series.
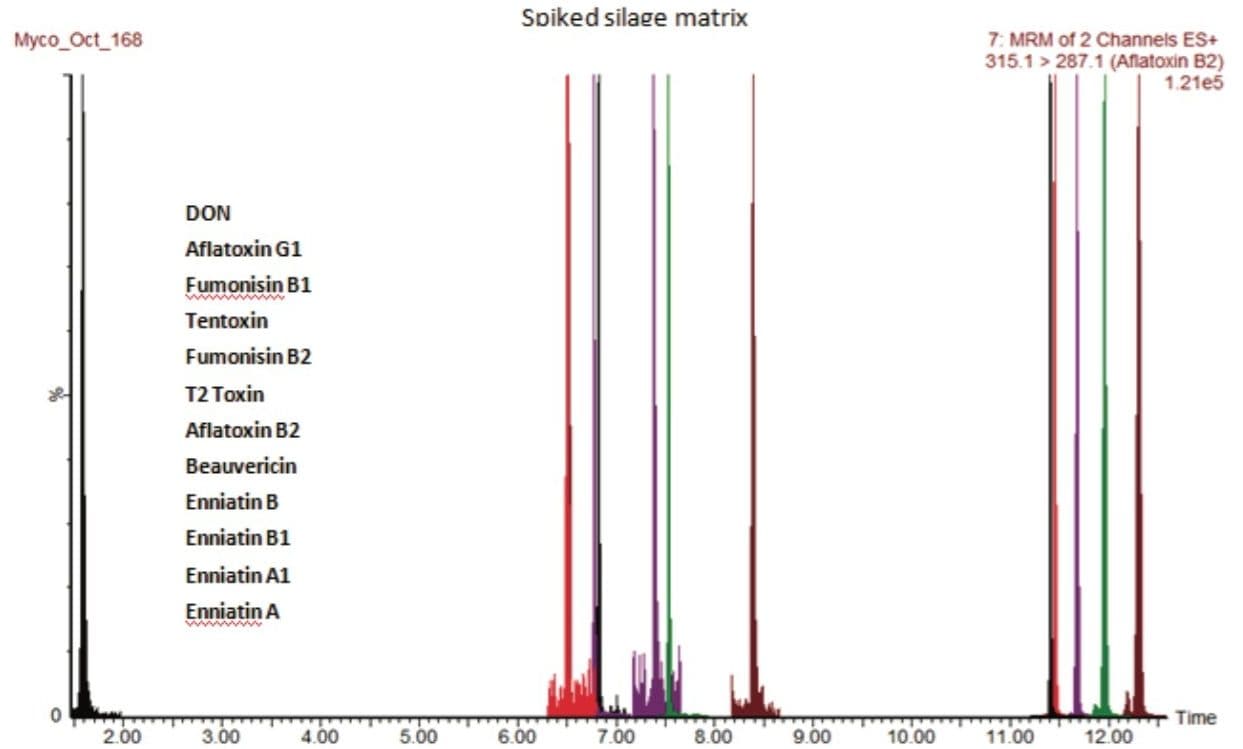
Fewer mycotoxins were detected in the silage samples (ranging from 0 to 3 of the 33 potential mycotoxins monitored for) by comparison with the compound feed samples. Figure 4 shows the chromatographic separation achieved for an extract of spiked silage following 1:10 dilution. The calculated concentrations ranged from circa 20 to 260 μg kg-1 The recovery values determined from the spiked control sample ranged from 89% to 108% for 17 mycotoxins with a mean value of 96%.

RIKILT, Institute of Food Safety, Wagnenigen, The Netherlands and the Ghent University, Ghent, Belgium for the provision of the animal feed and silage extracts and analytical standards.
720004961, March 2014