
The USP compendial method for ziprasidone HCl was run using ziprasidone standard and ziprasidone capsule formulated samples. The method was successfully transferred from HPLC to UPLC using the ACQUITY UPLC Columns Calculator and column chemistries that give identical selectivity independent of the particle size. The UPLC method is approximately 85% faster than the HPLC method with a 93% reduction in sample amount injected and solvent consumption, affording significant cost savings in routine batch analysis that is typical in Quality Control laboratories. The robustness of the L7 designated ACQUITY UPLC BEH C8, 1.7 μm particle column using traditional phosphate buffered mobile phases was evaluated using ziprasidone capsule sample injections on a UPLC system. This routine use evaluation demonstrated exceptional column stability of at least 2400 injections without compromising the system suitability specifications listed in the USP monograph.
USP compendial methods are routinely used as a basis for analyzing generic drugs. Column designations for USP methods will vary depending on the monograph. The columns described do not take advantage of sub-2 μm column technology and UPLC instrumentation, which can provide much greater efficiency in sample analysis. Here we demonstrate the method transfer from HPLC to UPLC for the USP compendial method for ziprasidone HCl, which designates an L7 column type.1
In this example, ziprasidone, an anti-psychotic drug, was analyzed in both standard and capsule forms. Since a USP method for the formulated drug is not available, the capsule was analyzed using the USP assay method for ziprasidone HCl. The assay suitability criteria described in the ziprasidone HCl monograph was used to evaluate successful method transfer. A routine use evaluation was also performed to further understand the effects of using phosphate buffered mobile phases and formulated drug samples on a sub-2 μm particle UPLC column.
|
Buffer: |
25 mM potassium phosphate, monobasic in water - pH 3.0 with potassium hydroxide |
|
Mobile Phase: |
60:40 buffer:methanol |
|
Separation Mode: |
Isocratic |
|
Detection: |
UV at 229 nm |
|
Column: |
XBridge C8, 4.6 x 150 mm, 5 μm (USP designation: L7), part number 186003017 |
|
Needle Wash: |
50:50 methanol:water |
|
Seal Wash: |
50:50 methanol:water |
|
Sample Diluent: |
60:40 methanol:water |
|
Flow Rate: |
1.5 mL/min |
|
Column Temp.: |
40 °C |
|
Injection Volume: |
20 μL |
|
Buffer: |
25 mM potassium phosphate, monobasic in water - pH 3.0 with potassium hydroxide |
|
Mobile Phase: |
60:40 buffer:methanol |
|
Separation Mode: |
Isocratic |
|
Detection: |
UV at 229 nm |
|
Column: |
ACQUITY UPLC BEH C8, 2.1 x 50 mm, 1.7 μm (USP designation: L7), part number 186002877 |
|
Needle Wash: |
50:50 methanol:water |
|
Seal Wash: |
50:50 methanol:water |
|
Sample Diluent: |
60:40 methanol:water |
|
Flow Rate: |
0.6 mL/min |
|
Column Temp.: |
40 °C |
|
Injection Volume: |
1.4 μL |
Empower 2 CDS
|
Tailing factor: |
not more than (NMT) 2.0 |
|
Replicate injections: |
NMT 2.0% RSD |
Ziprasidone capsules, contents made up to 0.23 mg/mL in diluent.
Ziprasidone standard, made up to 0.23 mg/mL in diluent.
Samples were filtered through a 0.2 μm PTFE membrane (P/N WAT200504) prior to analysis.
Samples were prepared by dilution to a concentration of 0.23 mg/mL, as described in the USP monograph for ziprasidone HCl. Since there is no monograph available for the capsule formulation, the contents of the capsule were prepared in the same manner as the ziprasidone standard. As an added step beyond the USP monograph, both samples were further filtered through a 0.2 μm PTFE membrane as a precautionary measure to remove any particulates prior to injection.
The USP monograph for ziprasidone HCl designates an L7 column and the suggestion listed is a Zorbax Rx-C8 column. An equivalent Waters L7 column, XBridge C8, was chosen using the Waters Column Selectivity Chart. This column chemistry is easily scaled to an ACQUITY UPLC BEH C8, 1.7 μm particle size column for UPLC analysis. The USP compendial method for ziprasidone HCl was first run as written using the XBridge C8 L7 designated column on an Alliance HPLC system. Five replicates of ziprasidone standard and ziprasidone capsule samples were analyzed. Assay suitability criteria, including USP tailing and injection repeatability, were found to be within specification for both samples (Table 1).
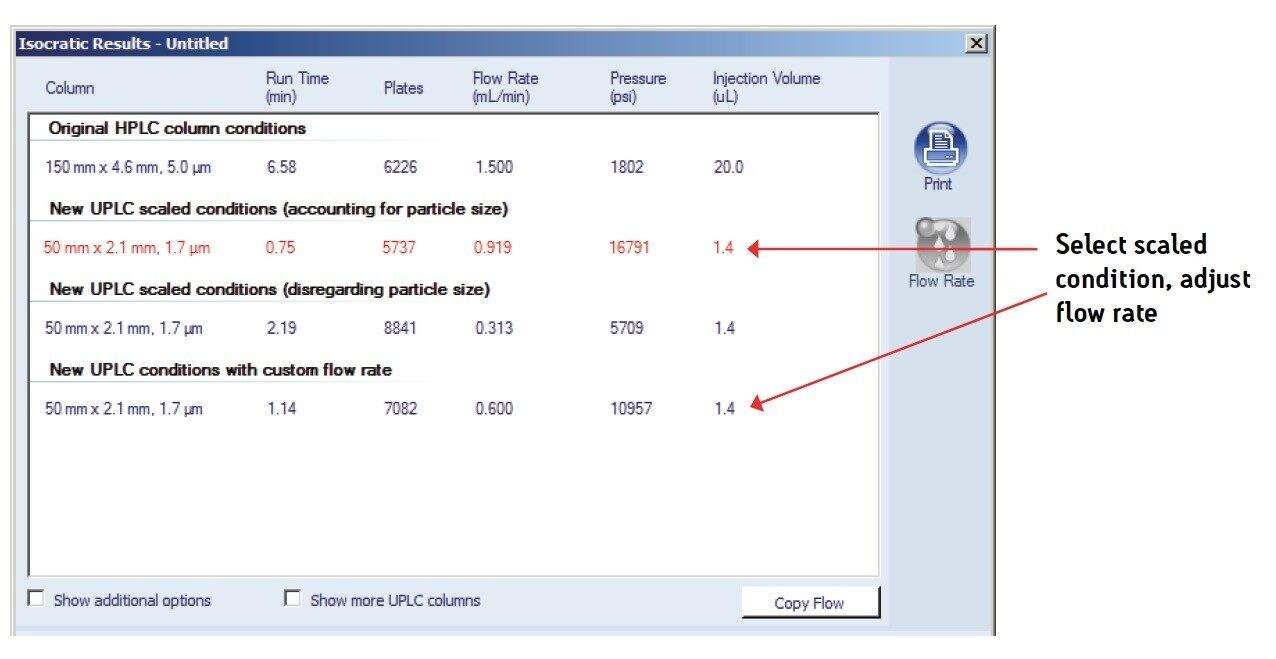
The USP method was then transferred from HPLC to UPLC using the ACQUITY UPLC Columns Calculator.2 The flow rate was adjusted for the scaled UPLC result as shown in Figure 1, to accommodate system pressure considerations. A comparison of chromatograms for ziprasidone capsules between HPLC and UPLC is shown in Figure 2. The UPLC method provides an approximately 85% faster run time (1.5 min UPLC vs 10 min HPLC), allowing for higher throughput of routinely analyzed samples. Additionally, mobile-phase solvent consumption and sample injected is also reduced by approximately 93% using the UPLC method.
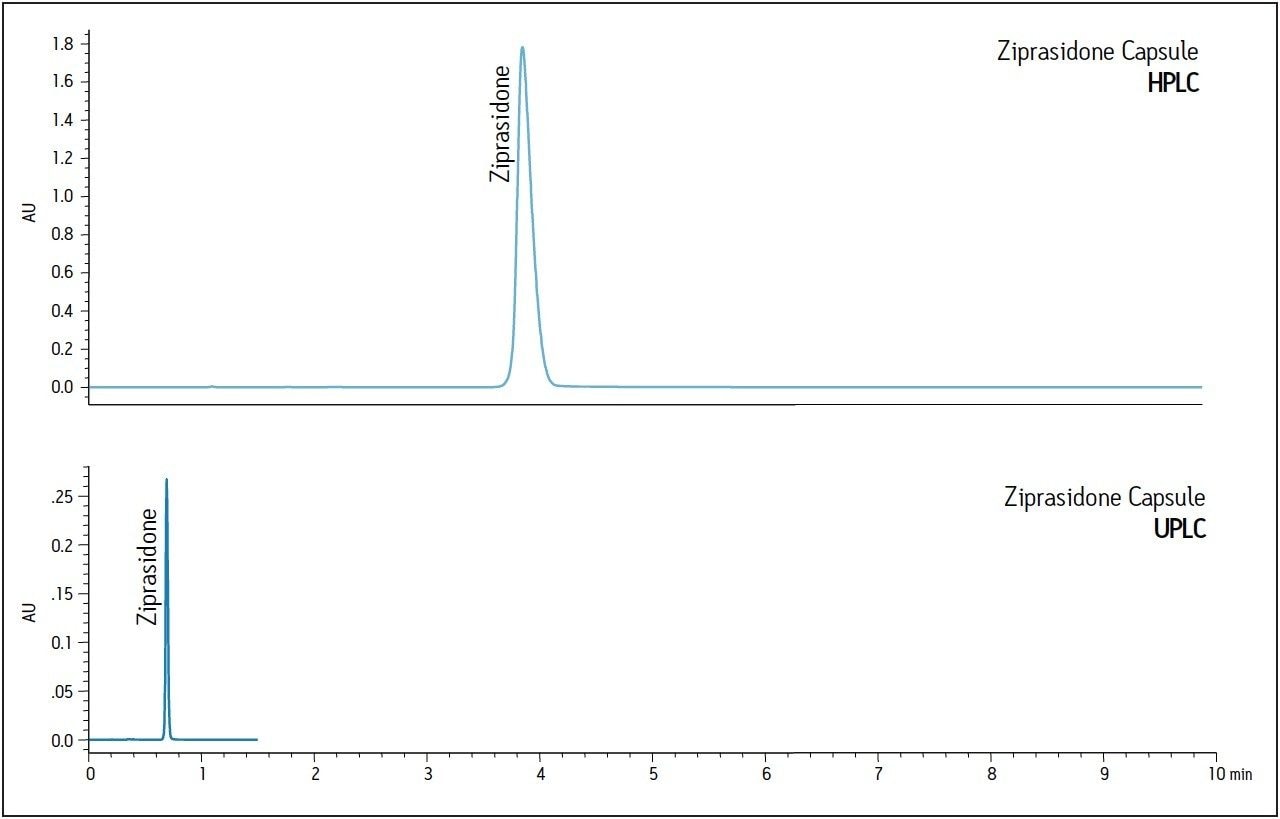
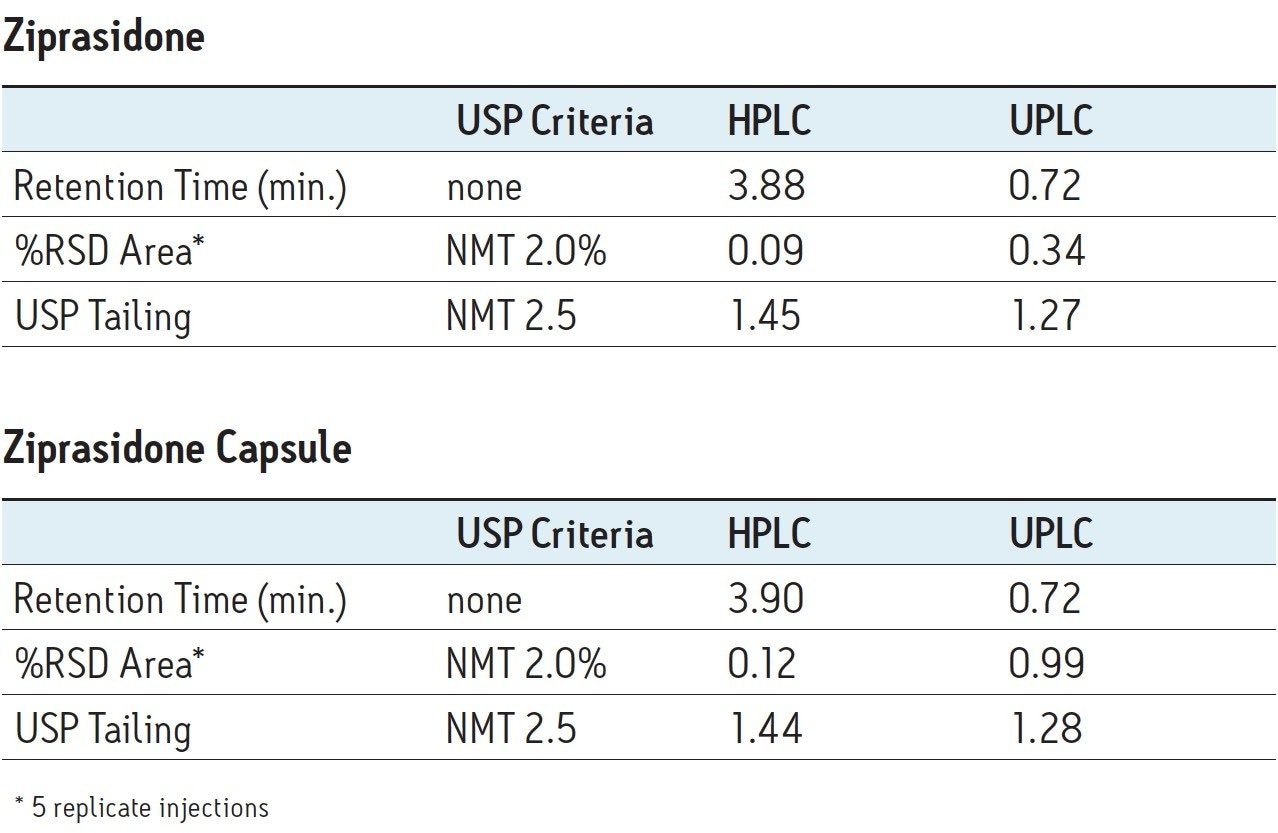
Assay suitability criteria including USP tailing and %RSD for peak area were compared for five replicate injections of ziprasidone standard and ziprasidone capsules on the HPLC and UPLC systems. Both systems were found to meet USP tailing and injection repeatability specifications as listed in the USP monograph for ziprasidone HCl, demonstrating a successful method transfer (Table 1).
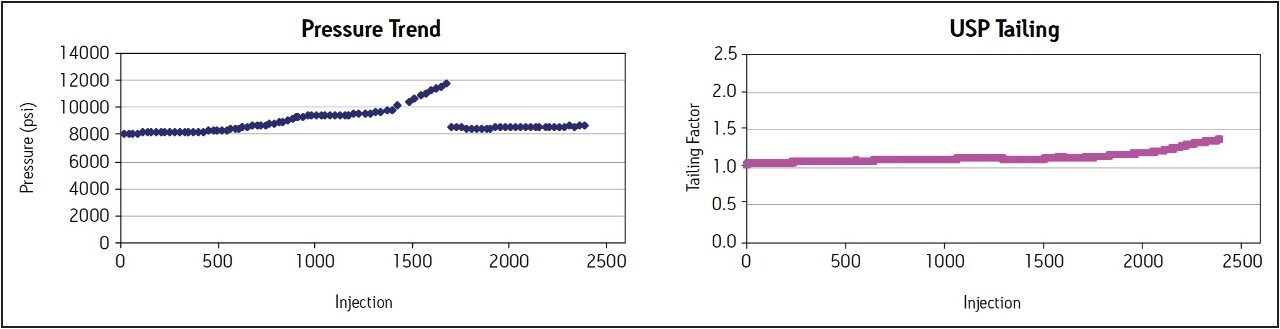
A routine use evaluation using the ACQUITY UPLC BEH C8, 1.7 μm particle column was performed to evaluate the effects of phosphate buffer and formulated drugs on L7 columns. One blank injection was followed by five replicate injections of the ziprasidone standard, followed by 20 replicate injections of the ziprasidone capsule sample and this sequence of injections was repeated continuously. Assay suitability criteria per the USP monograph for ziprasidone HCl were monitored throughout the study to determine when the column was no longer suitable to be used for analysis of the formulated drug.
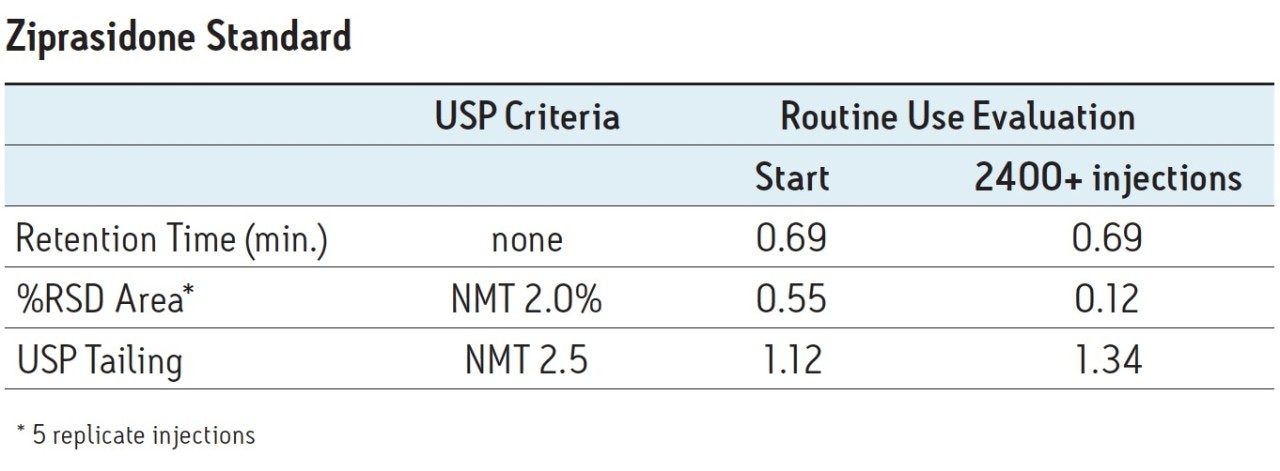
The routine use evaluation was performed for up to 2400 injections with no failures. The pressure and USP tailing factors remained stable throughout the study, as shown in Figure 3. After 2400 injections, assay suitability was evaluated on five replicate injections of the ziprasidone standard. All criteria passed well within the specifications listed in the USP monograph for ziprasidone HCl (Table 2), demonstrating column stability of at least 2400 injections using phosphate buffered mobile phases and repeated injections of a formulated capsule sample.
The USP compendial method for ziprasidone HCl was run using ziprasidone standard and ziprasidone capsule formulated samples. The method was successfully transferred from HPLC to UPLC using the ACQUITY UPLC Columns Calculator and column chemistries that give identical selectivity independent of the particle size. The UPLC method is approximately 85% faster than the HPLC method with a 93% reduction in sample amount injected and solvent consumption, affording significant cost savings in routine batch analysis that is typical in Quality Control laboratories. The robustness of the L7 designated ACQUITY UPLC BEH C8, 1.7 μm particle column using traditional phosphate buffered mobile phases was evaluated using ziprasidone capsule sample injections on a UPLC system. This routine use evaluation demonstrated exceptional column stability of at least 2400 injections without compromising the system suitability specifications listed in the USP monograph.
720004079, April 2013