This is an Application Brief and does not contain a detailed Experimental section.
This application brief describes the method to effectively and easily separate analyte(s) of interest from the phospholipid fraction present in plasma samples.
In bioanalysis, UPC2-MS/MS provides an orthogonal technique to RP-UPLC-MS/MS that offers an effective way to reduce or prevent matrix effects arising from coelution of phospholipids with hydrophobic compounds.
The reliability of analytical data upon which critical toxicological and efficacy findings are based is an essential part of bioanalysis. LC-MS/MS is the technique of choice in quantitative bioanalysis due to the high selectivity and sensitivity the method offers. However, quantitative analysis by LC-MS/MS is influenced by a phenomenon called matrix effects, wherein matrix components present in the biological samples can influence the MS response of the analyte under analysis and, therefore, produce erroneous results.
Residual matrix components, endogenous phospholipids in particular, are a significant source of matrix effects in plasma samples. To reduce or alleviate these effects, researchers have focused on optimizing sample preparation methods, manipulating chromatographic parameters, and varying MS modes of ionization.
One method to avoid potential phospholipid coelution and therefore potential matrix effects is to change the chromatographic selectivity of a separation. In reversed-phase (RP) chromatography, the relatively hydrophobic phospholipids require high percentages of organic solvent to elute from the column.
Thus, hydrophobic compounds of interest are prone to coelute with these lipid interferences and experience matrix effects.
UltraPerformance Convergence Chromatography (UPC2) utilizes supercritical carbon dioxide as the major mobile phase, resulting in vast selectivity differences when compared to standard reversedphase LC in bioanalysis.
A precursor scan of m/z 184 was employed to monitor elution of phospholipids, as this MS method detects the most prevalent phospholipids found in plasma samples, including lysophosphatidylcholines and phosphatidylcholines (Figure 1). Clopidogrel is an example of a compound that, under typical reversed-phase UPLC conditions, coelutes within the phospholipid region (Figures 1A and B).
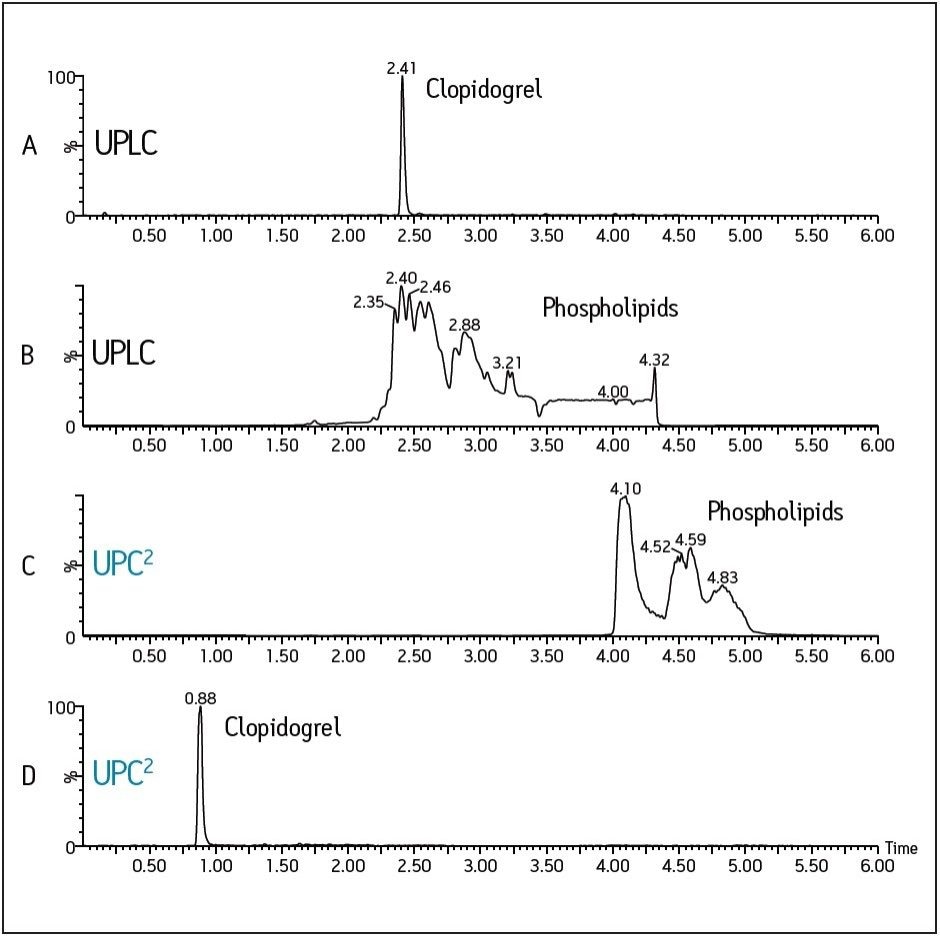
Clopidogrel was extracted from human plasma using a 3:1 protein precipitation. The reversed-phase UPLC sample was run on an ACQUITY UPLC BEH C18 Column, employing water and acetonitrile modified with ammonium hydroxide as the mobile phase. The same sample was then injected onto the ACQUITY UPC2 System using an ACQUITY UPC2 BEH Column and a CO2/methanol gradient, with ammonium hydroxide as the additive to methanol.
Figures 1C and D clearly demonstrate that the retention of clopidogrel decreases from 2.41 in RP-UPLC to 0.88 minute in UPC2. However, the phospholipids still elute toward the end of the gradient. Using UPC2, this example demonstrates how hydrophobic drugs like clopidogrel can be effectively separated from the phospholipid elution region with minimal method development.
Due the orthogonality of UPC2 with RP-UPLC, the analyte of interest elutes much earlier away from the interfering phospholipids, minimizing the chance of matrix interferences and ensuring more accurate and precise quantification of dirty samples.
Using UPC2, it is possible to easily and effectively separate compounds of interest (i.e., hydrophobic drugs like clopidogrel) from coeluting phospholipids. This was achieved with minimal method development time or advanced sample preparation techniques. The addition of UPC2 in a bioanalytical laboratory provides an orthogonal technique that offers an effective way to reduce or prevent matrix effects arising from coelution of phospholipids with hydrophobic compounds.
720004707, May 2013