
This is an Application Brief and does not contain a detailed Experimental section.
This application brief describes the rapid and direct chromatographic analysis of formulated lithographic products using ACQUITY UltraPerformance Convergence Chromatography (UPC2). ACQUITY UPC2 provides near real-time assessment to direct product manufacture correction as needed, and facilitates product composition control to help eliminate mischarges and product escapes.
Lithographic materials such as photoresist and anti-reflective coatings are formulated specialty coatings produced for the electronics industry. These two coatings are often used in combination to minimize notching and maintain line width control when processing on highly reflective substrates resulting in improved pattern transfer. Dyed photoresist is often employed to combine both the properties of anti-reflective coatings with the lithographic properties of the photoresist in a single package. Many products in this family utilize aromatic azo dyes, such as the Sudan dyes family.1,2,3
Differences in dye assay, dye solubility, and other manufacturing variations result in a need for product analysis to enable corrective adjustments to dye concentration. Current analysis involves a functional evaluation with UV measurements of cast film, which yield bulk values without specific component information. As a result, added functional testing is required which can increase manufacturing cycle time and test cost in excess of $2,000 per batch. Additional normal phase HPLC testing is commonly employed to drive batch correction for mischarges. HPLC test cycle times are 12 to 24 hours, and involve extensive sample preparation, including polymer precipitation and filtration.

In this technology brief, the analysis of formulated products using Waters ACQUITY UPC2 System – based on the principles of supercritical fluid chromatography – is described. The final formulated product was diluted ten-fold with tetrahydrofuran, loaded into a sample vial, and directly injected – without the need for extensive sample preparation, such as polymer precipitation, filtration, or lengthy system equilibration.
Chromatograms from the mixed standard solution, a blank non-dyed product and the fully formulated product with the mixed dye package are shown in Figure 2. The analysis was conducted in less than two minutes, and provided baseline resolution of the four dyes, as well as the typical dye impurities.
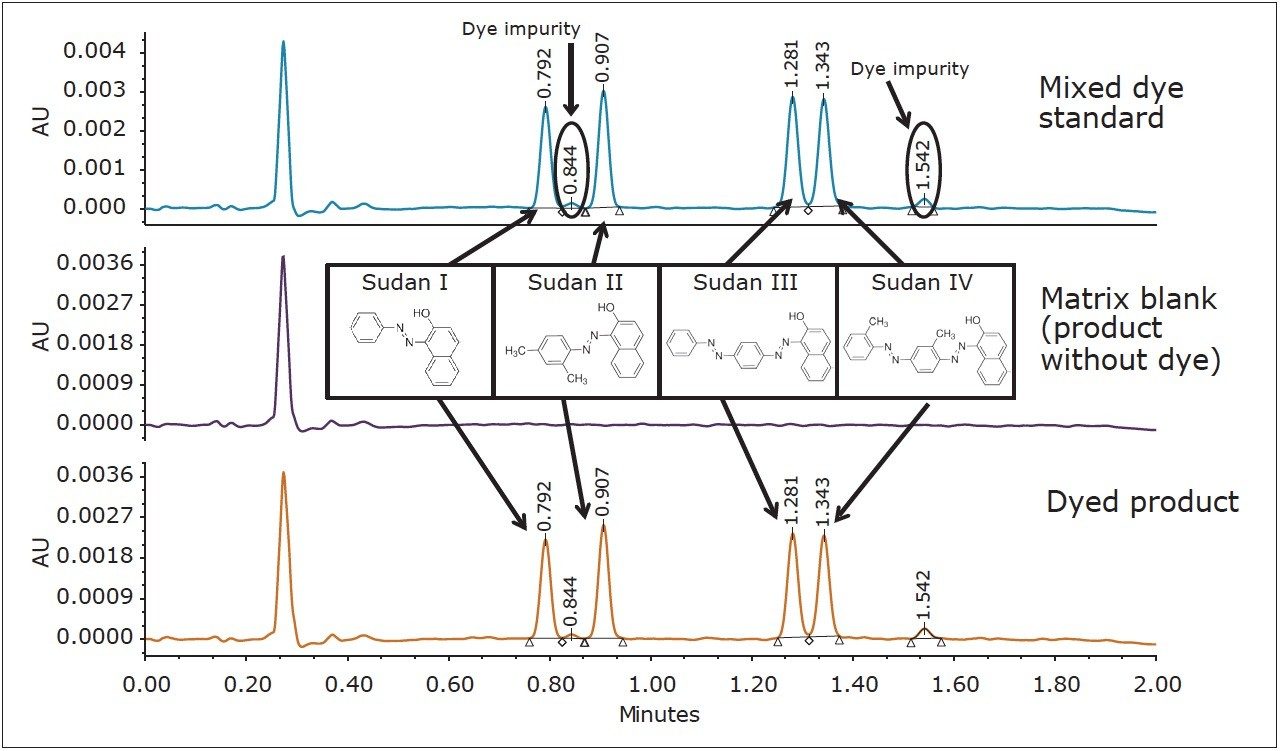
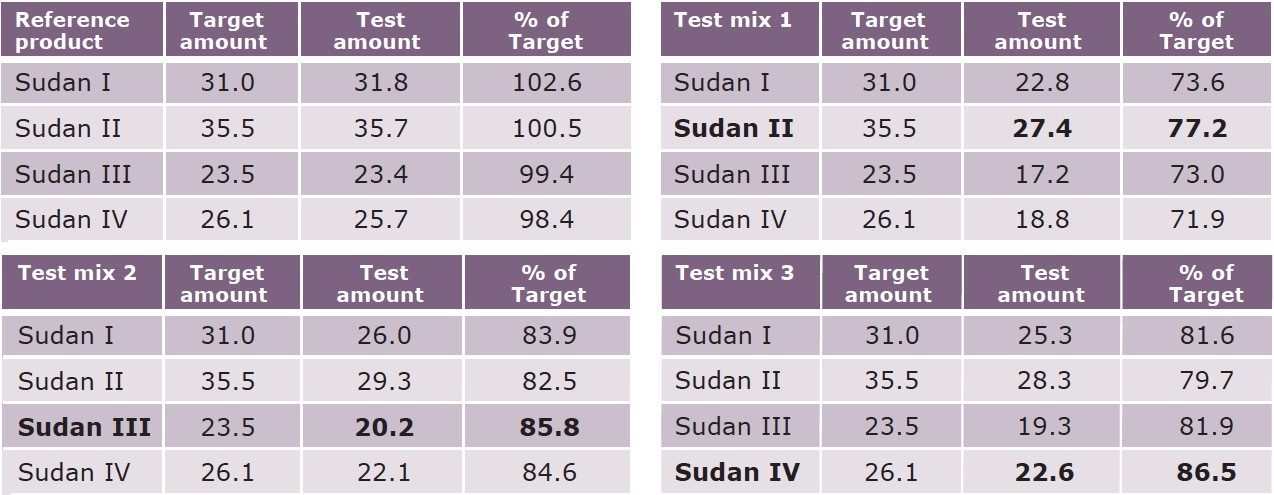
Quantification of the analytes in the product was easily accomplished using an external calibration based on mixed dye standards. The data from the analysis of four product mixes is presented in Table 1. The analysis of the reference product provides the quantitative verification of the product dye mixture as compared to target or formulation amounts. Examples of in-process testing of three test mixes showed a low loading of the dye package. Additionally, the analysis highlighted the disproportionate level of individual dyes in the formulated dye blend in each product. Using this analysis, a formulation correction to each product mix can directly address the total dye level, as well as the relative dye proportions in a fraction of the time of traditional approaches.
720004393, June 2012