
The analysis and isolation of polar compounds from natural products is often challenging because of the difficulty in retaining hydrophilic compounds on a reversed-phase column. Hydrophilic Interaction Chromatography (HILIC), is an orthogonal chromatographic separation technique which separates hydrophilic compounds by their interaction with a polar stationary phase. In this application note, we demonstrate the utility of the BEH Amide particle for the analysis and isolation of a stevia-related compound in a commercially-available sweetener.
BEH Amide columns are specifically designed to enhance the retention of polar compounds by HILIC, making analysis, scaling, and isolation of these molecules easy.
Although reversed-phase is usually the chromatographic mode of choice for many samples, extremely polar compounds are often difficult to analyze because they do not retain on non-polar columns. Hydrophilic Interaction Chromatography (HILIC) is a complementary technique that utilizes a polar stationary phase to bind hydrophilic compounds. Analytes are separated based on a unique combination of liquid-liquid partitioning, adsorption, ionic interaction and hydrophobic retention mechanisms. Compounds elute from the column as the gradient transitions from low aqueous to high aqueous mobile-phase composition. The BEH Amide column, with a trifunctionally-bonded amide phase, was first introduced in 2009 with 1.7 μm particles for the analysis of polar compounds using the ACQUITY UPLC System. Demand for a column capable of analyzing and isolating compounds such as saccharides, synthetic sugars, glycopeptides, and polar compounds from natural products has driven the development of a larger 5 μm particle for use in preparative HPLC applications. In this application note, we demonstrate the utility of the BEH Amide particle for the analysis and isolation of a stevia-related compound in a commercially-available sweetener.
|
System: |
Waters 2525 Binary Gradient Module, 2767 Sample Manager, Column Fluidics Organizer, 2996 Photodiode Array Detector, ZQ 2000 Mass Spectrometer, and 2420 Mass Detector |
|
Columns: |
XBridge BEH Amide, 5 μm, 4.6 x 150 mm, part number 186006595 XBridge BEH Amide, 5 μm, 19 x 150 mm, part number 186006605 |
|
Column Temp.: |
25 °C |
|
Mobile Phase A: |
80/20 acetonitrile/water with 0.1% ammonium hydroxide |
|
Mobile Phase B: |
30/70 acetonitrile/water with 0.1% ammonium hydroxide |
|
Weak Needle Wash: |
75/25 acetonitrile/water |
|
Strong Needle Wash: |
20/80 acetonitrile/water |
|
Seal Wash: |
50/50 acetonitrile/water |
|
Sample Diluent: |
50/50 acetonitrile/water |
|
Flow Rate: |
Reported in figures |
|
Gradient: |
Reported in figures |
|
Injection Volume: |
Reported in figures |
1.2 mg of commercially-available stevia sweetener was dissolved in 1 mL 50/50 acetonitrile/water. The sample mixture was vortexed and filtered through a 13 mm, 0.45 μm GHP syringe filter, part number WAT200516.
832 mg of commercially-available stevia sweetener were dissolved in 20 mL 50/50 acetonitrile/water. The sample mixture was vortexed and filtered through a 25 mm, 0.45 μm GHP syringe filter, part number WAT200514.
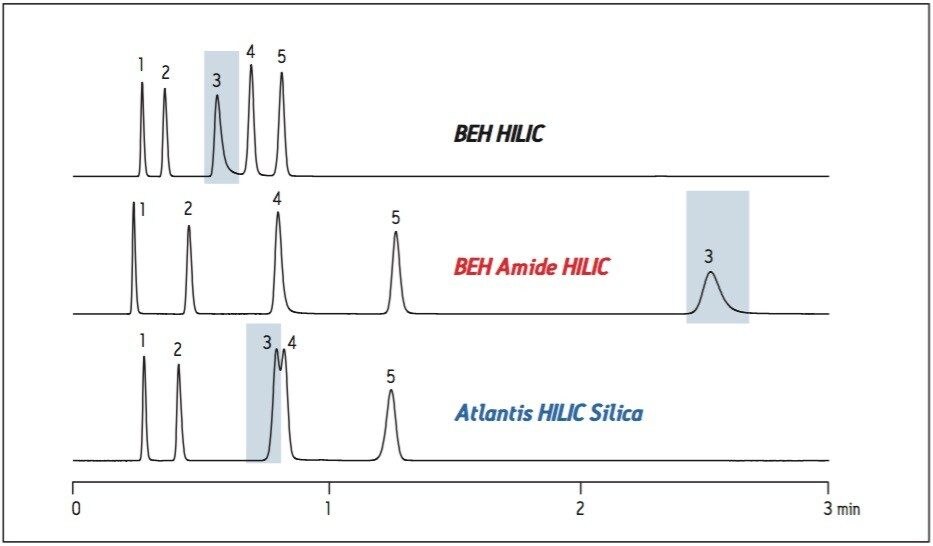
The analysis and isolation of polar compounds from natural products is often challenging because of the difficulty in retaining hydrophilic compounds on a reversed-phase column. Hydrophilic Interaction Chromatography (HILIC), is an orthogonal chromatographic separation technique which separates hydrophilic compounds by their interaction with a polar stationary phase. Liquid-liquid partitioning, adsorption, ion exchange, and hydrogen bonding mechanisms all contribute to the retention of the sample. Analytes are eluted from the column by increasing the polarity of the mobile phase. The selectivity and retentivity of compounds on different stationary phases is dependent upon the specific properties of the column packing. As shown in Figure 1, the selectivity and retentivity of the analytes are different for each of the three HILIC stationary phases when the column dimensions and the chromatographic method are held constant. The BEH Amide column shows the most retention for the various types of compounds and a different selectivity compared to the other two columns.
Although polar compound retention is the primary reason for employing HILIC chromatography, the sample diluent also plays a role in retention, influencing sample solubility and peak shape. Traditional unbonded HILIC stationary phases usually require diluents and mobile phases with high organic concentration which limit the solubility of polar compounds at the high sample concentrations used in prep chromatography. Small amounts of water, even 10-20%, make the injection solvent incompatible with initial HILIC conditions on unbonded phases. Since the BEH Amide bonded phase tolerates mobile phases and injection solvents which are higher in aqueous content, polar compounds like sugars and peptides can be solubilized at concentrations amenable to preparative chromatography. In addition, because of the higher organic content employed in HILIC in general, the mass spectrometer response is usually enhanced for ionizable compounds and the fraction drying time is reduced.
Stevia rebaudiana Bertoni is an herb that grows as a small shrub in the mountainous regions of Paraguay and Brazil. The natural sweetness of stevia is attributed to stevioside and rebaudioside, two polar glycosides isolated from the leaves of the stevia plant. Stevioside and rebaudioside are about 300 times sweeter than sugar, yet they are calorie- and carbohydrate-free. These properties alone help to promote Stevia’s acceptance for use in food products.
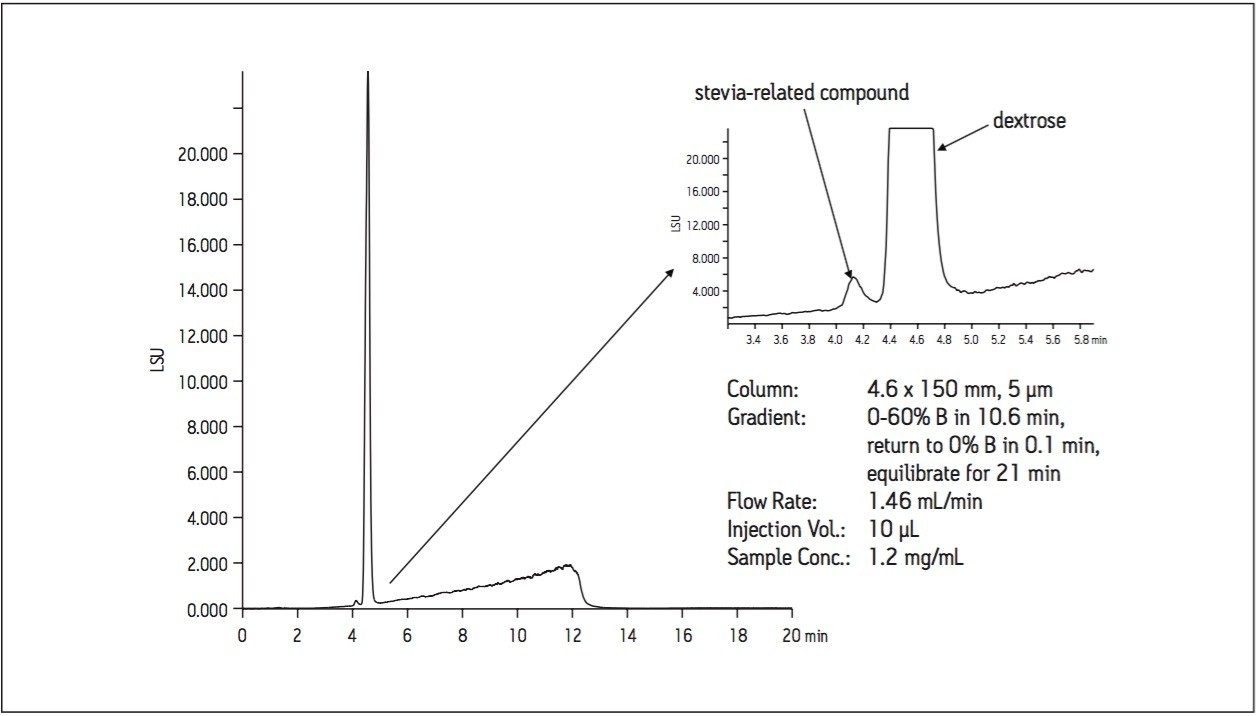
As shown in Figure 2, the analysis of a commercially-available stevia sweetener on a 5 μm, 4.6 x 150 mm BEH Amide column indicates the presence of a minor component eluting prior to the main peak. Upon further investigation, it was determined that the stevia-related compound is actually the minor component in the formulation. To better understand the composition of a compound mixture, scientists often isolate minor components for identification. Repetitive injections of the sample are usually required to effectively isolate an appropriate amount for subsequent analyses.
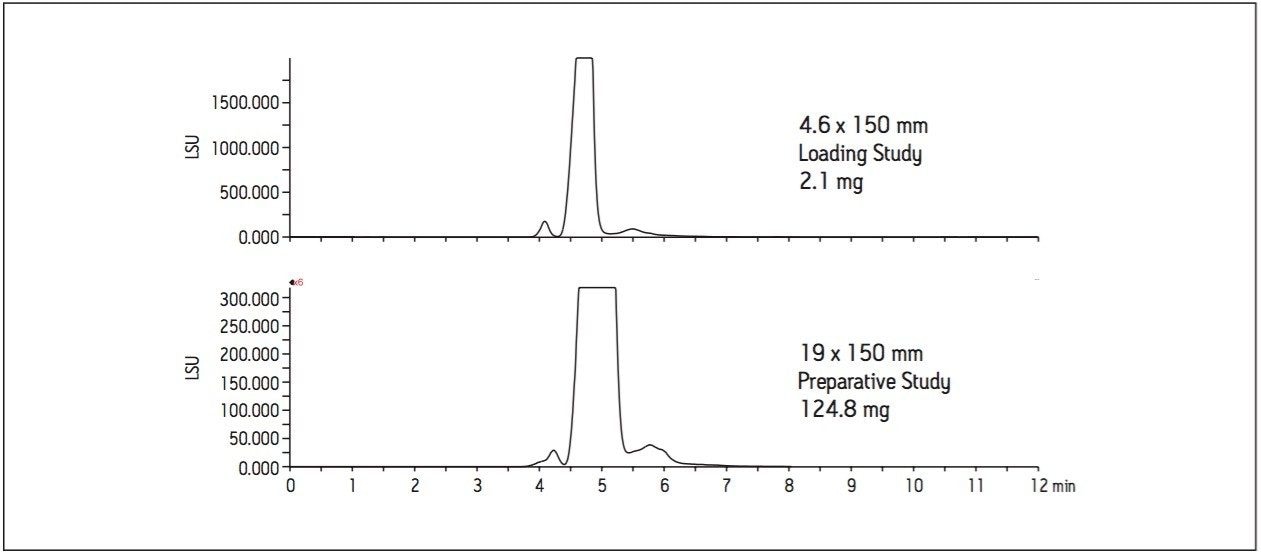
A loading study was performed at the small scale to determine the sample mass that could be loaded on the column without compromising the resolution. Improved peak resolution leads to higher mass capacity and better purity for isolated compounds. Figure 3 illustrates the results of a loading study performed on the analytical column with the maximum injection volume of 50 μL, equivalent to 2.1 mg of sample on column.
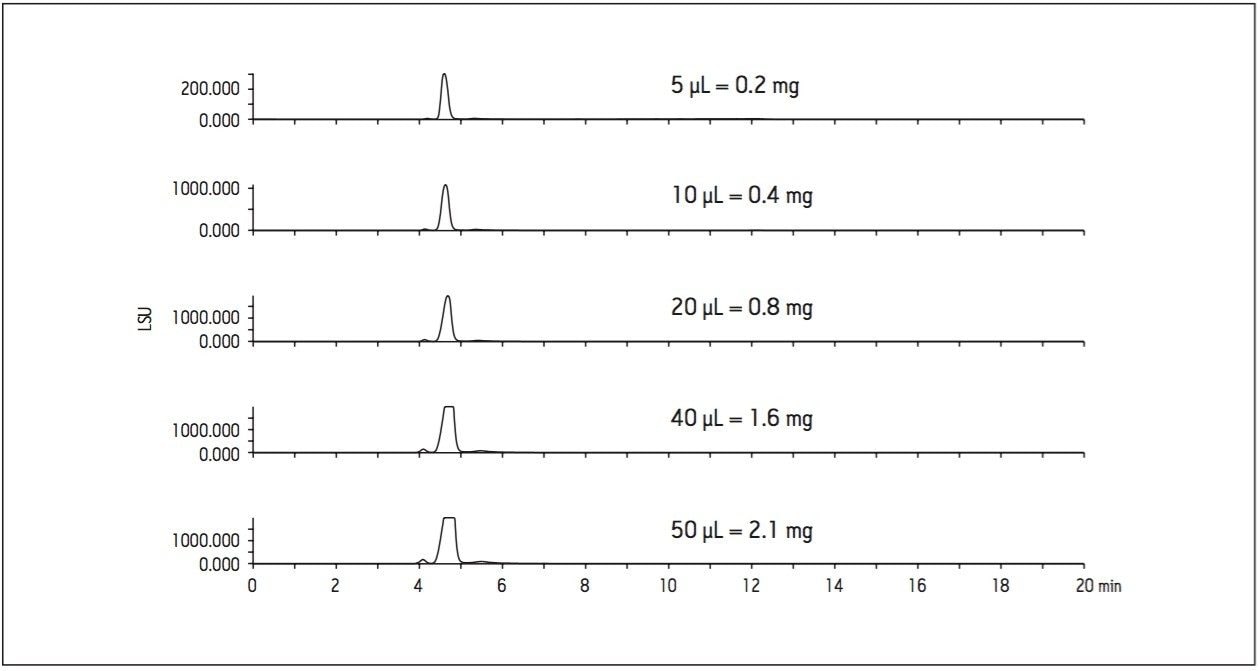
A sample mass of 2.1 mg on the analytical column scales to about 36 mg on a 19 mm preparative column, but excellent resolution between the minor component and the larger main peak suggested that an even higher load on the 19 mm preparative column would be acceptable.
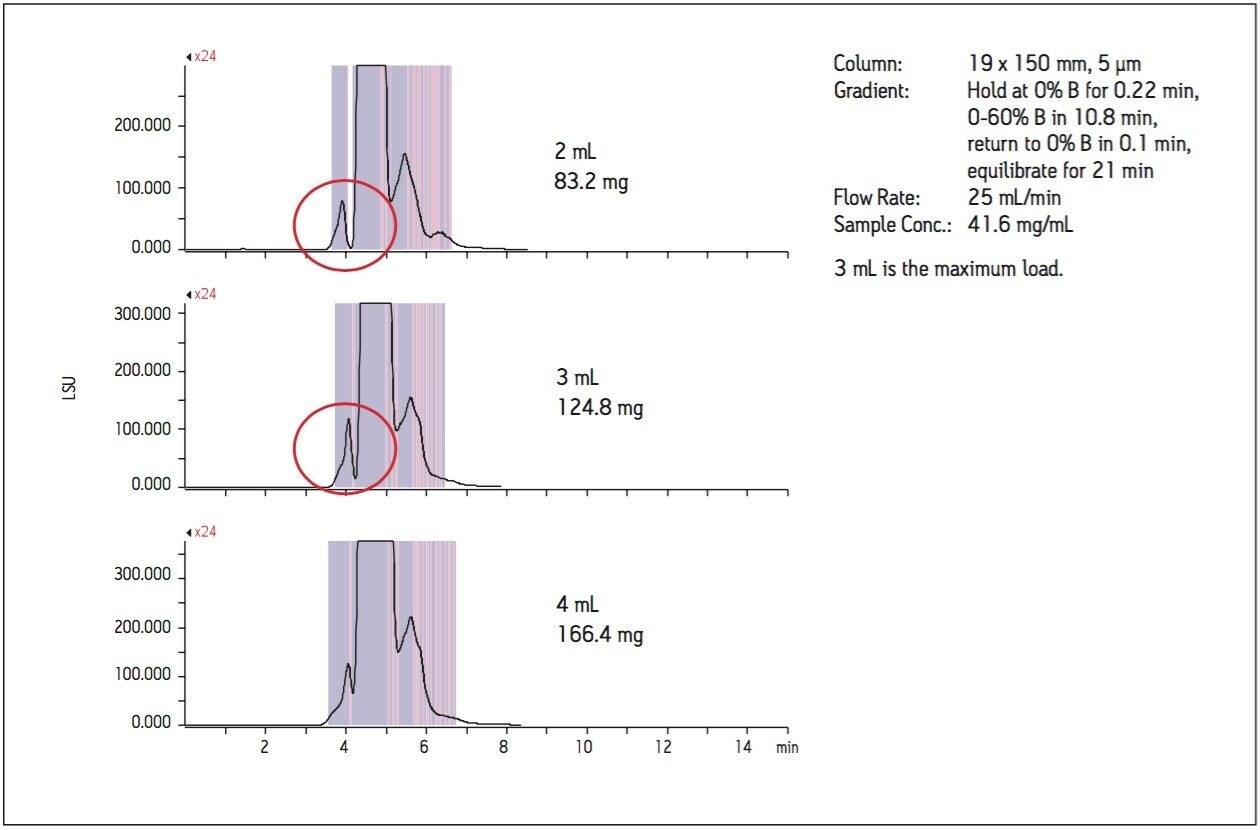
Without further investigation, the separation method was scaled to the 19 x 150 mm column. As shown in Figure 4, 2 mL of the sample mix, equivalent to about 83 mg on column, gives excellent resolution between the minor component and the main peak. The 3 mL injection volume with a load of about 125 mg also indicates that this larger mass capacity can be successfully applied to the column with reasonable resolution between the minor component of interest and the main peak. While a load of 166 mg can be injected on the preparative column, the loss of resolution between the peaks of interest can reduce the purity of the isolated peaks. The mass capacity on the BEH Amide column in this study is comparable to the estimated mass capacity on a reversed-phase column with the same dimensions.
Scaling separations requires matching column chemistry as well as appropriately scaled gradients. A properly-scaled separation gives the same chromatographic profile at increased mass capacity as the chromatographic profile at the smaller scale. Figure 5 illustrates the BEH Amide column scalability by comparing the chromatography using the small-scale gradient at maximum load with the large-scale chromatography used for the isolation. Note the baseline resolution between the minor component eluting at about 4 minutes and the main peak.
720004284, April 2012