In this study, we demonstrate detection of leuprolide in human plasma at very low concentrations using a Waters ACQUITY UPLC and Xevo TQ-S tandem quadrupole system, which is able to deliver the required sensitivity for this challenging assay.
A unique optimization of this Waters system for bioanalysis, from sample preparation to UPLC chromatography to tandem quadrupole MS, enables the determination and quantification of leuprolide at the 5 pg/mL level.
Leuprolide is a synthetic nonapeptide that acts as an agonist at pituitary GnRH receptors. Leuprolide down-regulates the secretion of gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) by interrupting the normal pulsatile stimulation and the desensitization of the GnRH receptors.
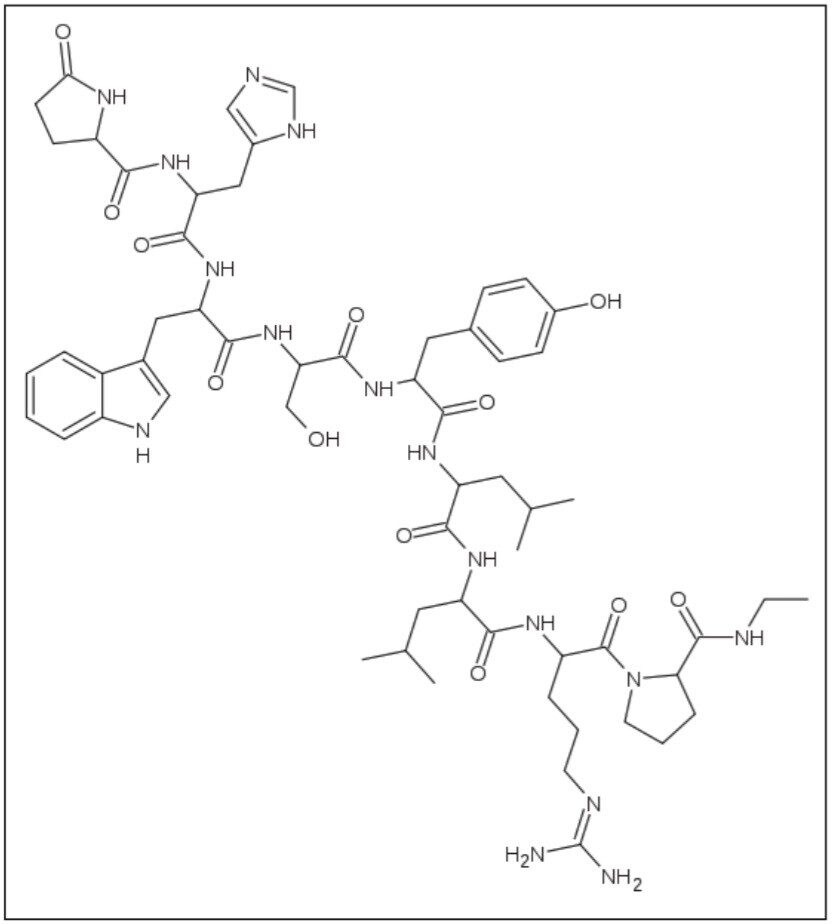
An LH-RH (GnRH) analog, leuprolide (Figure 1) may be used in the treatment of hormone-responsive cancers such as prostate cancer or breast cancer, estrogen-dependent conditions (such as endometriosis or uterine fibroids) to treat precocious puberty, and also to control ovarian stimulation in in vitro fertilization (IVF). It is also considered a possible treatment for paraphilias.
Leuprolide received its first approval for treatment of advanced prostate cancer in 1985 and received several other approvals for treating prostate cancer at several dosage levels. However, monitoring leuprolide and its analytes in its low-dosage concentration had not been possible because analytical techniques were not able to offer the necessary sensitivity.
In this study, we demonstrate detection of leuprolide in human plasma at very low concentrations using a Waters ACQUITY UPLC and Xevo TQ-S tandem quadrupole system, which is able to deliver the required sensitivity for this challenging assay.
|
LC system: |
ACQUITY UPLC System equipped with a Binary Solvent Manager, Column Manager, and Sample Manager |
|
LC column: |
ACQUITY UPLC BEH 300 C18, 2.1 x 100 mm, 1.7 μm |
|
Gradient: |
Reversed-phase chromatography with an acidic aqueous buffer solution and acetonitrile as the organic modifier |
|
Elution: |
30-95% organic gradient over 3.5 min |
|
LC gradient run: |
5.0 min starting with 90% acid solution and 10% acetonitrile |
|
Column temp.: |
50 °C |
|
MS system: |
Xevo TQ-S |
|
MS mode: |
Positive ion electrospray MS/MS |
|
MS transition: |
605.69 ⇒ 249.11 |
When compared to an HPLC, UPLC Technology enables a user to achieve faster separations with lower consumption of the mobile phase. UPLC also offers short run times with low dispersion, resulting in better separation of signals from unwanted signals arising from plasma, phospholipids, and other endogenous materials. In addition, the ACQUITY UPLC BEH 300 Column used in this study offers significantly better separation of peptides when compared to traditional reversed phase columns.
For mass spectrometry, the Xevo TQ-S offers the advantage of high sensitivity and its ZSpray Technology provides added stability to the analysis. The combined effect of the outstanding UPLC column chemistry and chromatographic system along with the Xevo TQ-S provides a powerful tool for the estimation of a variety of molecules, from small molecules to large peptides, in very low concentrations.
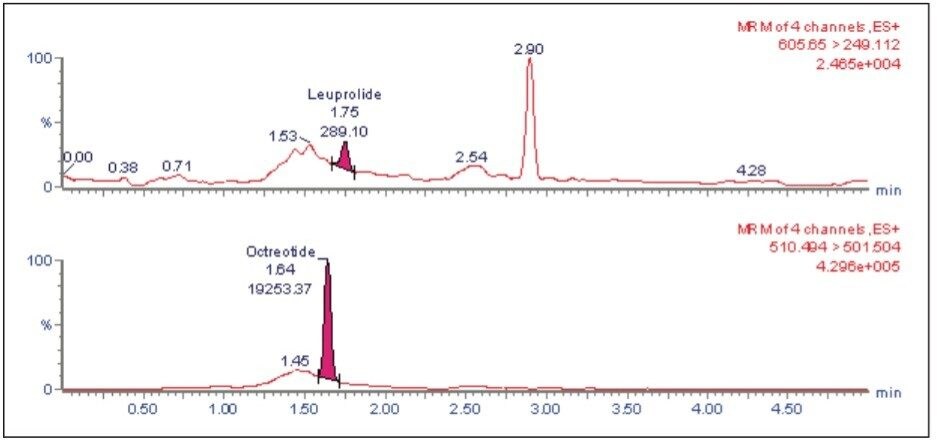
The leuprolide analyte eluted with a retention time of 1.75 min. As can be observed from Figure 2, the leuprolide signal shows excellent symmetrical peak shape and resolution from endogenous interferences. Such excellent peak shape and chromatographic resolution can be attributed to an optimized combination of the ACQUITY UPLC System and BEH 300 C18 Column.
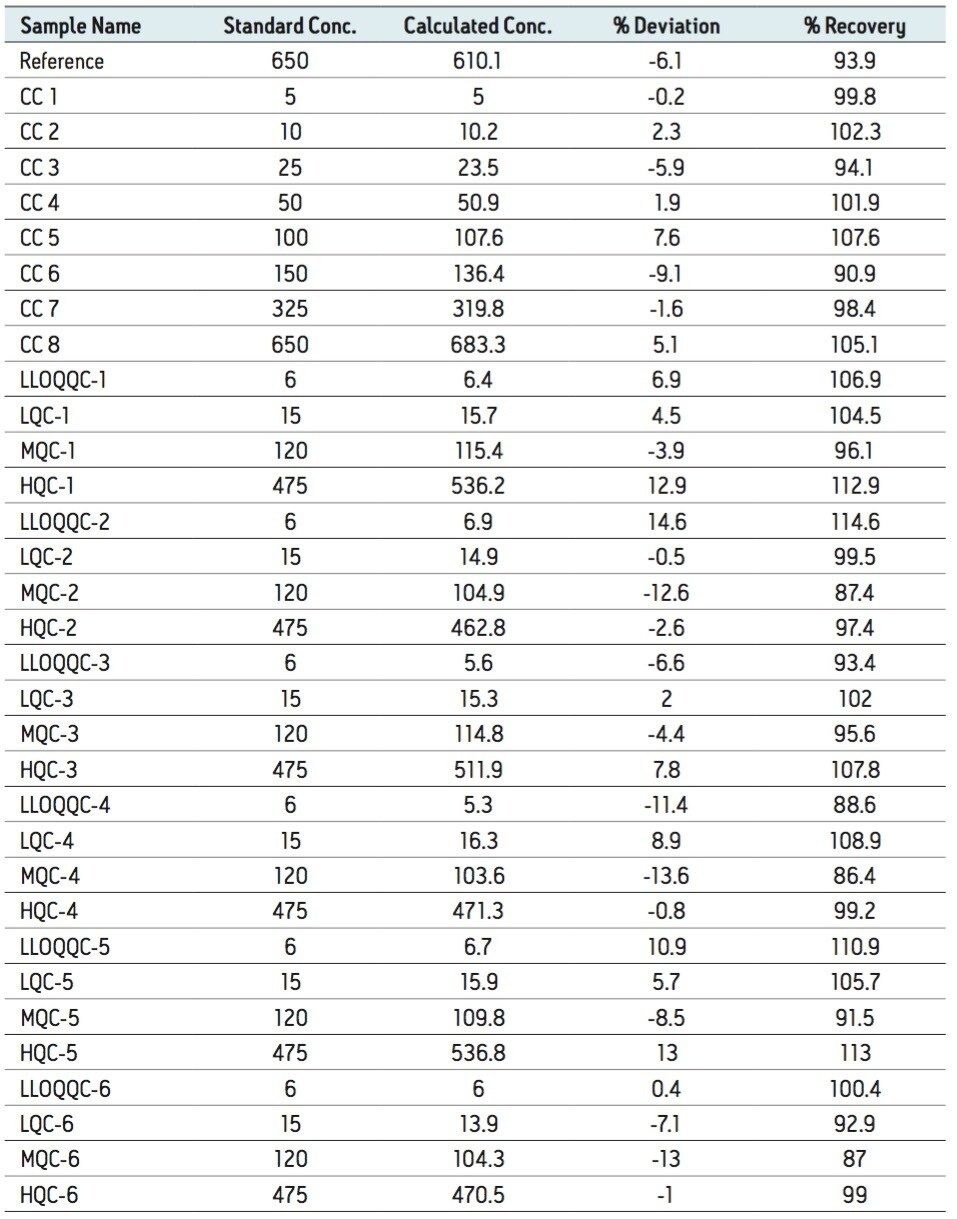
The LLOQ for leuprolide was determined to be 5 pg/mL with the signal-to-noise ratio being at least 20:1 (Figure 2). In addition, the results showed excellent reproducibility at LLOQ levels (Table 1). Such excellent reproducibility and sensitivity provides the ability to quantify leuprolide at the LLOQ level.
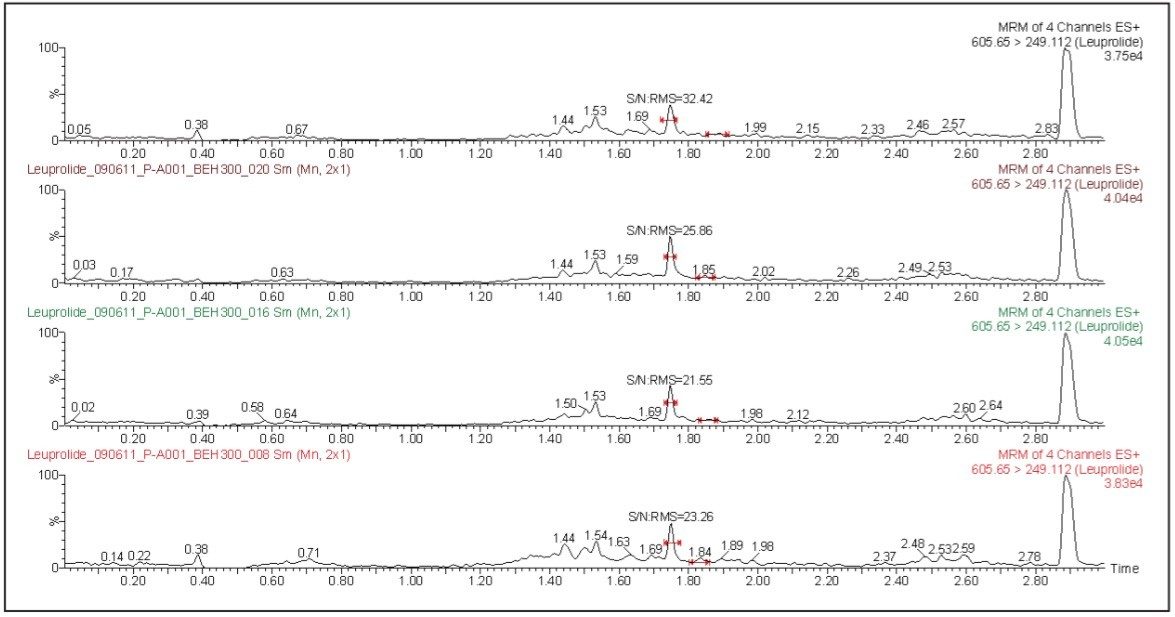
The LLOQ obtained for this analysis was due, in part, to the high-sensitivity detection provided by the Xevo TQ-S System. The co-joined, off-axis StepWave ion guide in the Xevo TQ-S provides superior levels of sensitivity while maintaining the robustness and cleanliness of the source and ion optics. This enables the Xevo TQ-S to increase the ion flux entering the mass spectrometer, resulting in the highest levels of sensitivity. The assay reported in this study was demonstrated to be linear over the range of 5 to 650 pg/mL in both organic solvents and human plasma samples.
As observed from Table 1, the %RSD varied from 4.887 to 6.874 within a range of concentration. Although the inter-day precision varied between 2.130 and 9.475 for the lowest level of concentration, the medium,high-concentration ranges and the LLOQQC showed a variation within the range of 5.128 to 9.927. In addition, the percent recovery of samples that was conducted by comparing the area under the curve of the extracted sample with that of the neat sample, was found to be about 60% at all concentration levels.
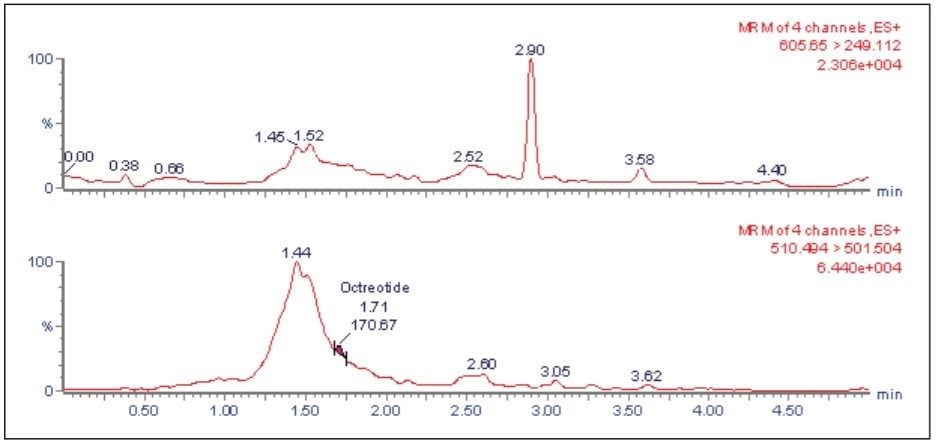
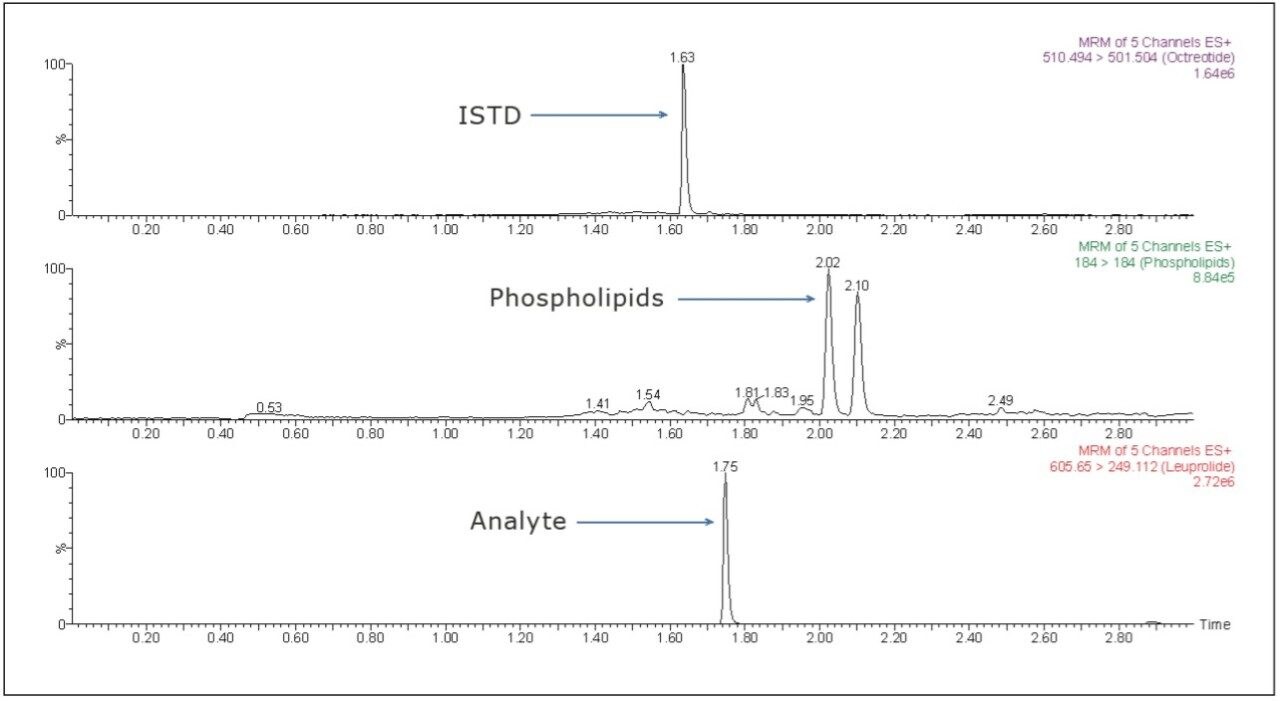
The LC-MS/MS chromatogram of the blank plasma sample and that of the leuprolide at LLOQ level showed little interference from the endogenous materials (Figure 3 and Figure 4). In addition, phospholipid elution was checked by injecting an extracted standard with an MS scan at 184 ⇒ 184 m/z, a unique MRM transition for typical phospholipids. As can be observed from Figure 5, no significant phospholipid elution was observed at the retention time of either the leuprolide analyte (1.75 min) or the internal standard, octreotide (1.63 min).
Matrix interference, which is considered to be one of the major regulatory challenges in the pharmaceutical world, is automatically calculated by MassLynx Software. A comparison of the post-spiked samples with those of the neat samples in the same concentration level reveals that the matrix interference for the Leuprolide samples was not more than 2.051%. Such a value is well within the acceptable range indicated by the regulatory agencies.
The low circulating concentration levels of leuprolide, a synthetic nonapeptide that acts as an agonist at pituitary GnRH receptors, requires a highly sensitive assay for accurate determination of the pharmacokinetics in humans, especially in prostate cancer patients.
This study demonstrates that the combination of Oasis SPE Technology, the ACQUITY UPLC System with a BEH 300 C18 column, and the Xevo TQ-S Mass Spectrometer combine to enable the development of an assay for Leuprolide with an LLOQ of 5 pg/mL in human plasma.
The UPLC chromatograms demonstrate not only better chromatography, but also better resolution compared to the data obtained from any conventional HPLC systems. The assay reported in this study showed excellent reproducibility, specificity, and robustness. Despite the complicated nature of this analytical challenge, the overall cycle time for the UPLC-MS/MS experiments reported in this study was about 3 minutes.
720004094, August 2011