This is an Application Brief and does not contain a detailed Experimental section.
Targeted peptide quantification in complex biological samples requires an analytical LC-MS system with a wide dynamic range. This application brief presents the quantification of EGFR phosphopeptides using the SYNAPT G2 HDMS System.
The SYNAPT G2 HDMS System can quantify phosphopeptides with limits of quantification in the low attomole range and quantitative dynamic range over four orders of magnitude.
LC-MS is increasingly being used for peptide and protein quantification to elucidate biological processes and to identify and verify candidate biomarkers. The use of time-of-flight for qualitative studies is well accepted and there are inherent advantages in terms of mass resolution and accuracy. However, for high-accuracy quantification, the use of MRM assays on tandem quadrupole mass spectrometers has traditionally been required to obtain quantitative measurements over a wide dynamic range. The SYNAPT G2 HDMS System provides new capabilities for quantitative measurements, due to the high-dynamic range of the detection system that can provide accurate measurements of target components over a wide range of ion intensities, irrespective of spectral complexity.
The EGFR phopshopeptide GSTAENAE(pY)LR was spiked from 100 attomoles to 2 pmoles into a constant background of BSA tryptic peptides. Peptides were separated using a Waters nanoACQUITY UPLC System coupled to a SYNAPT G2 HDMS System. Each sample was analyzed in duplicate by LC-MSE an unbiased TOF acquisition switching between low and elevated energy on alternate scans. Detection of the [M+2H]2+ ion for the EGFR phosphopeptide m/z 645.8 could be determined at a retention time of 20.2 min, even at the lowest level (100 attomole injected), due to the high resolution and accuracy afforded by the TOF, as shown in Figure 1. In the case of the 100 attomole spectrum the in-spectrum dynamic range was approximately 6000, highlighting the wide dynamic range of the oa-TOF mass analyzer. LC-MSE fragment ion data, shown in Figure 2, allowed positive identification of the peptide and site of post translational modification.


The purpose of this study was to determine whether a linear response could be obtained for the phosphopeptide over a wide concentration range. Figure 3 shows the quantification curve obtained for the area under the MS peak for the 2+ molecular ion. A linear response is obtained from 100 attomoles to 2 pmoles on-column (four orders of magnitude) with R2 values of better than 0.99. Duplicate injections are shown in Figure 3 where it can clearly be seen that excellent reproducibility was obtained, without any normalization or internal standard correction.
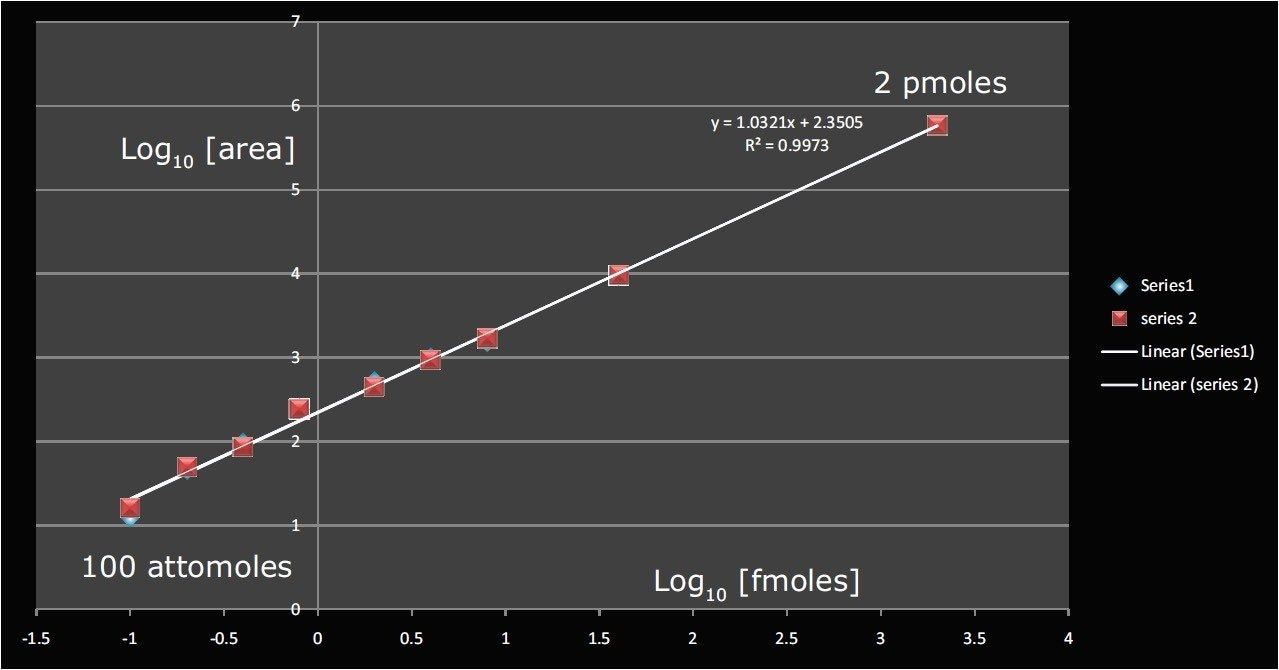
Here we have shown quantification of the EGFR phosphopeptide by the SYNAPT G2 HDMS System with limits of quantification in the low attomole range and quantitative dynamic range over four orders of magnitude. These results are very similar to those obtained by MRM on a tandem quadrupole system, but the TOF approach has the additional benefit that due to the full-scan, untargeted nature of the experiment, large numbers of compounds can be screened using the specificity afforded by accurate mass and high-resolution within a single UPLC run.
720003474, May 2010