This application note shows how tandem quadrupole mass spectrometry has been employed for the isolation of the metabolites of common pharmaceuticals from urine. The application of different modes of data acquisition, including scan, MRM, constant neutral loss, and precursor ion is demonstrated. We also demonstrate how the use of MS/MS directed purification facilitates the combination of samples from several chromatographic runs.
The identification of drug metabolites following animal or human volunteer studies is essential to drug discovery and development as well as the regulatory submissions process. Traditionally, this has been achieved by the use of liquid or gas chromatography coupled to mass spectrometry.1,2 More recently, the use of hyphenated techniques such as LC/NMR and LC/NMR/MS have become more commonplace in the drug metabolism laboratory, allowing a more precise identification of the site of metabolism.3,4
While LC/NMR and LC/NMR/MS are extremely powerful tools, they are typically low throughput and limited in sensitivity. The capacity of analytical columns restricts the amount of material that can be loaded onto the column before the column exhibits either volume or mass overloading effects and the chromatographic resolution is lost. Thus, LC/NMR is less attractive for the analysis of highly potent compounds dosed at low levels or those compounds that undergo extensive metabolism. In such cases, it is often necessary to perform a pre-concentration step, such as SPE or liquid/liquid extraction, both of which are time consuming and run the risk of losing of valuable information.
The use of MS-directed purification, using semi-preparative scale columns (typically 19 mm I.D.), is now commonplace within the pharmaceutical industry, especially to support lead candidate purification. This approach has also been applied to the isolation of drug metabolites with some success.5 The extra sensitivity and selectivity of MS/MS mass spectrometry allows for more precise selection of drug metabolites. Furthermore, the use of neutral loss and precursor ion scanning detection modes facilitates the collection of drug metabolites without the need for prior knowledge of compound metabolism. The Application of MS/MS Directed Purification to the Identification of Drug Metabolites in Biological Fluids Paul Lefebvre, Robert Plumb, Warren Potts, and Ronan Cleary Waters Corporation, Milford, MA, USA.

A Waters Alliance HT System was used with a SunFire C18 5 μm 4.6 x 100 mm Column at 40 °C. Eluent flow was split 1:20 with a Valco tee. 95% of the flow passed the 2996 Photodiode Array (PDA) Detector to the Fraction Collector III. The other 5% of the flow was routed directly to the Quattro micro Mass Spectrometer equipped with an ESCi multi-mode ionization source.
Water/acetonitrile in 0.1% formic acid, 1.25 mL/min total flow gradient. 0 to 5 min: 0%; 5 to 35 min: 0% to 10% B; 35 to 35.5 min: 10% to 95% B; 35.5 to 39.5 min: 95% B; 39.5 to 40 min: 95% to 5% B; 45 minutes end.
Electrospray positive, 3 kV capillary voltage, 30 V cone voltage, 20 V collision energy (for MS/MS experiments).
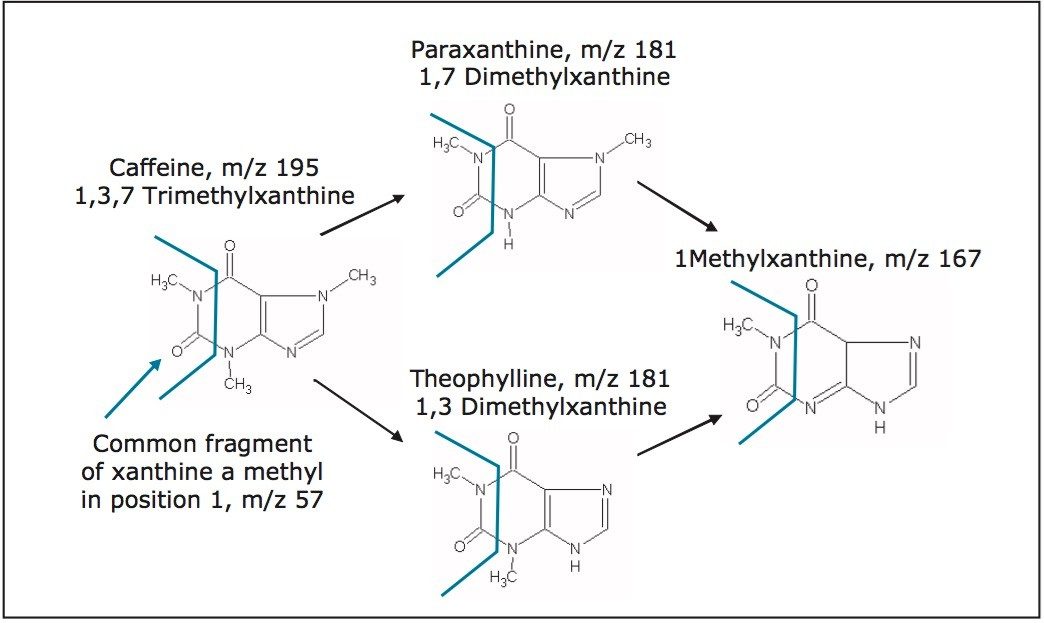
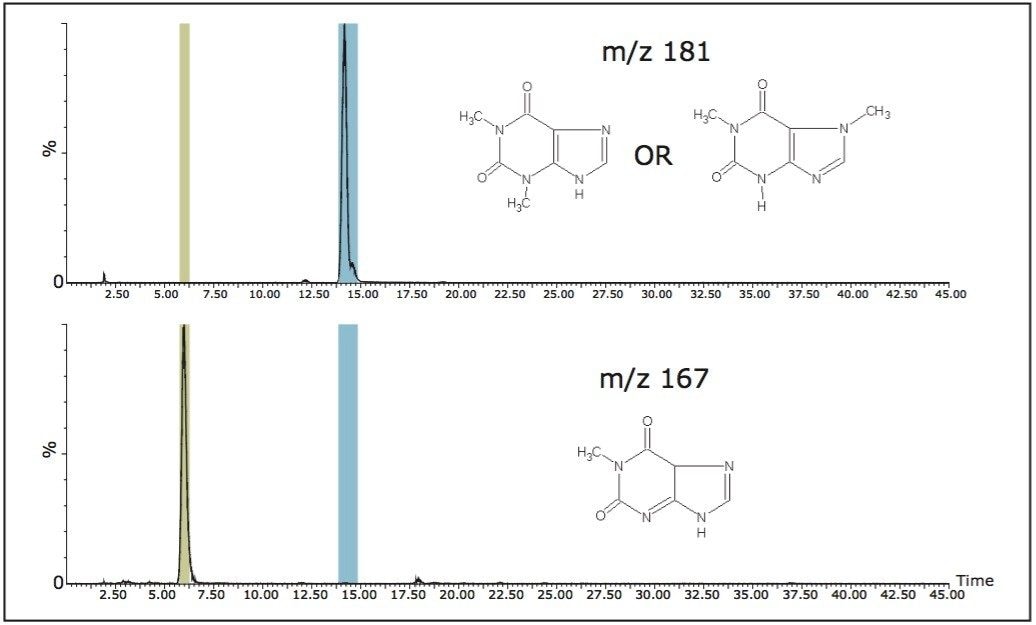
Figure 2 shows a portion of the caffeine metabolism pathway by demethylation.6 Target metabolites maintain the methyl group in position 1. They also have a common fragment ion, m/z 57.
Water/acetonitrile/10 mM ammonium formate, 1.25 mL/min total flow gradient. 0 to 5 min: 5%; 5 to 35 min: 5% to 60% B; 35 to 35.5 min: 60% to 95% B; 35.5 to 39.5 min: 95% B; 39.5 to 40 min: 95% to 5% B; 45 minutes end.
Electrospray negative, 3 kV capillary voltage, 30 V cone voltage, 20 V collision energy.
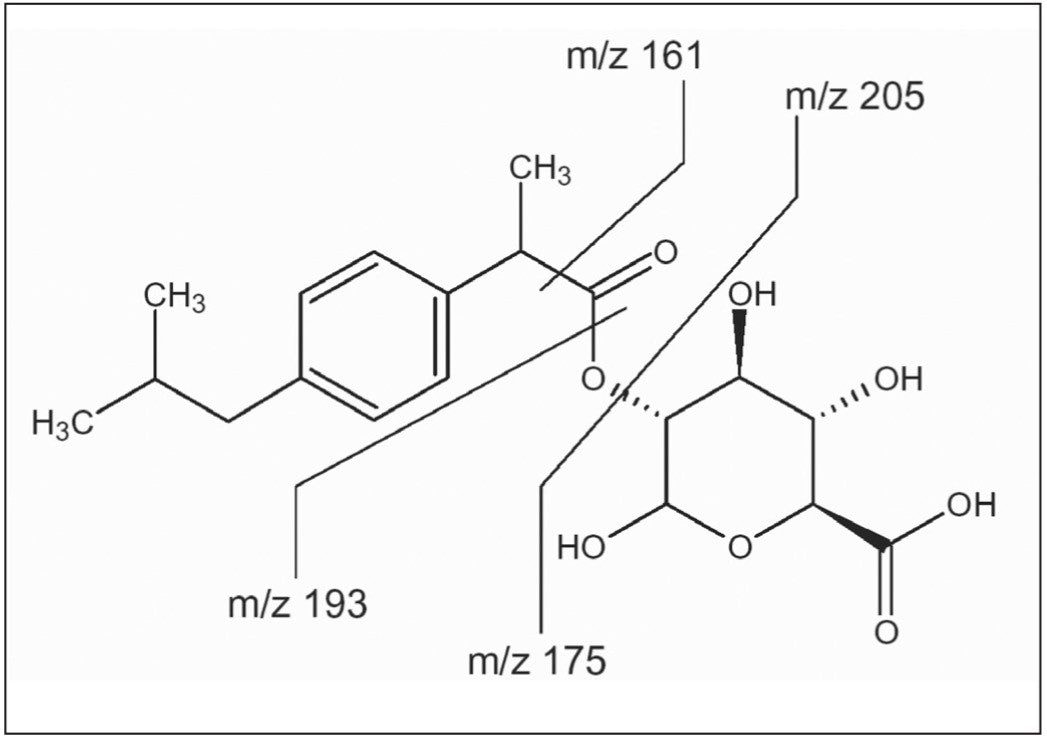
Figure 3 shows the fragmentation patterns of the ibuprofen gluceronide metabolite.7
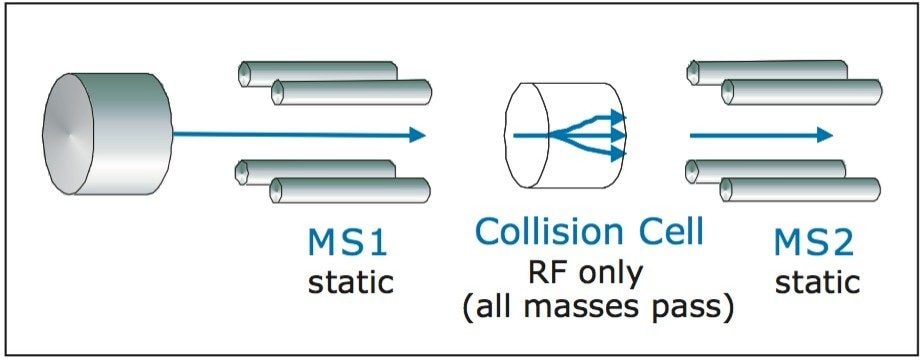
With single quadrupole directed purification, all ions generated in the source are passed through the quadrupole and detected. This is possible on the Quattro micro Mass Spectrometer by using the scan mode of acquisition. Only MS1 is scanned and there is no collision energy or scanning of Q3.
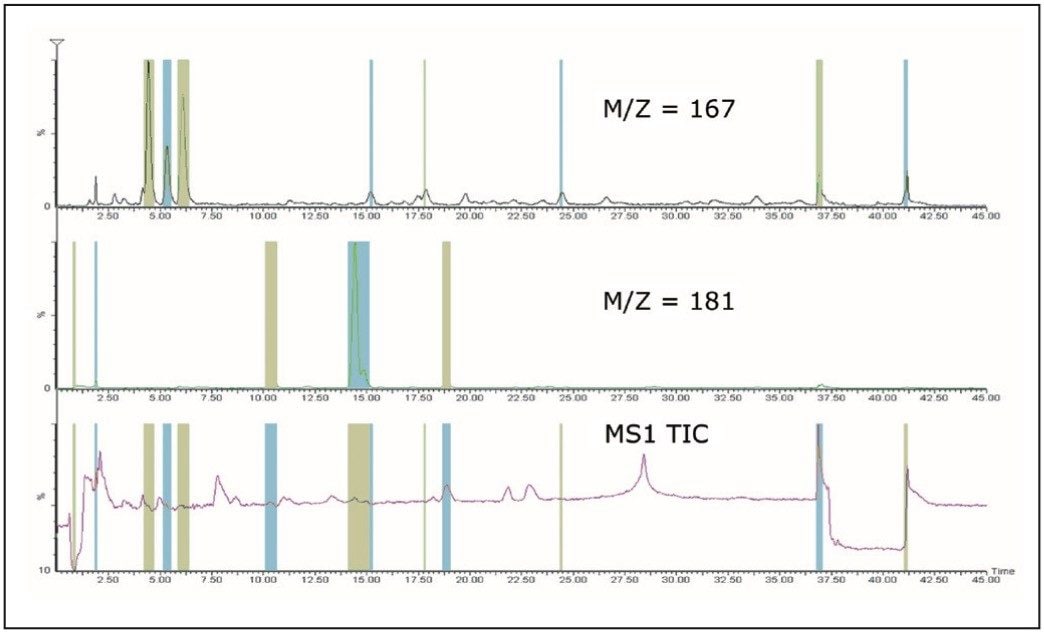
Because all of the ions generated are detected in this mode, complex mixtures can contain numerous isobaric interferences. Consequently, multiple fractions can be generated from a single m/z value. Figure 4 shows the collection of the caffeine metabolites with m/z 167 and 181 detected using only the first quadrupole. There are eight fractions collected for m/z 167 and five fractions collected for m/z 181, with additional analysis required to determine the fraction of interest.
With multiple reaction monitoring (MRM) data acquisition, MS1 is pre-selected on the precursor mass and MS2 is preselected on a specific product ion, as illustrated in Figure 5.
By selectively detecting a product ion, the signal-to-noise ratio is optimized, thus reducing the isobaric interference and allowing only the target to be collected. This mode of acquisition requires previous knowledge of the exact precursor and the exact product ions before purification.
Figure 6 shows the MRM acquisition and collection of the caffeine metabolites. The metabolites of interest for isolation have the transitions of 181 to 134, and 167 to 110.
For a peak to be present in the MRM chromatogram, both the specific precursor and the specific product ion need to be detected. For each target, only one fraction was collected.
A second possible mode of fraction triggering is from constant neutral loss acquisition. Here both MS1 and MS2 are scanned in synchronization, as illustrated in Figure 7. When MS1 transmits a specific precursor ion, MS2 looks for a product that is the precursor minus the neutral loss value. If the correct product is present, it registers at the detector. The constant neutral loss spectrum shows only the masses of all the precursors that lose the specific mass.
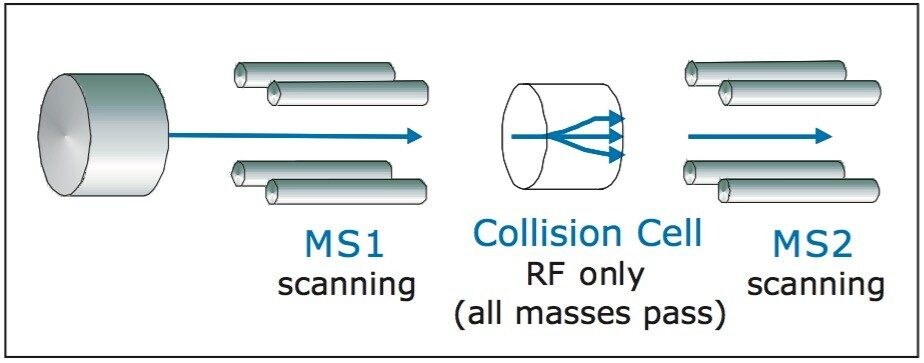
Figure 8 displays the constant neutral loss of 57 acquisition and collection of the caffeine metabolites with m/z 167 and 181. It shows that two fractions are collected, one for each mass. These fractions contain the target mass and have the specific neutral loss.
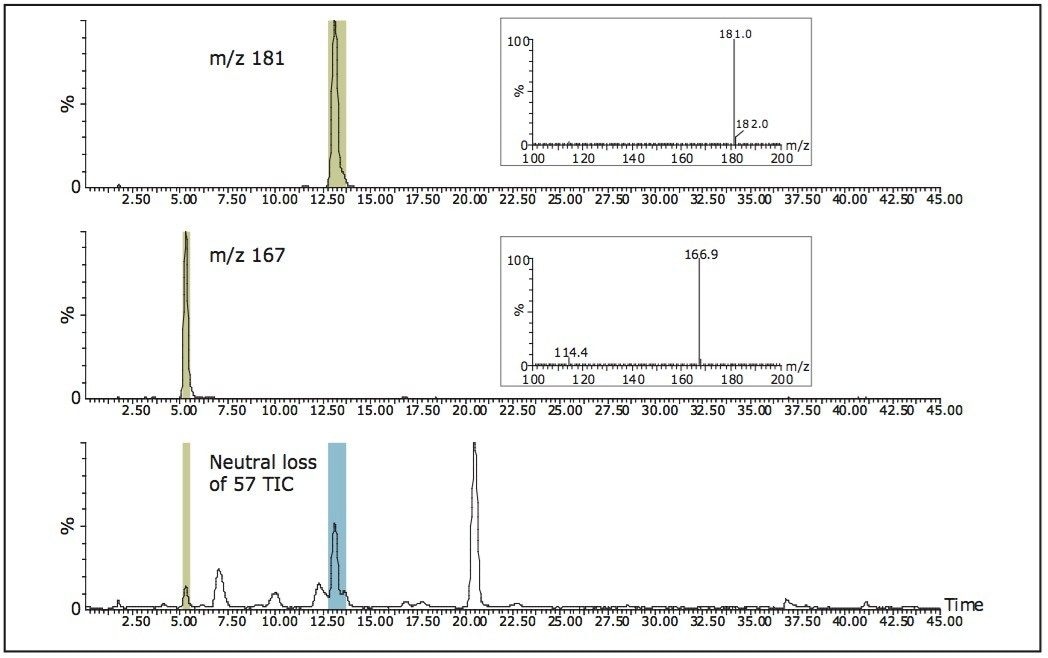
With constant neutral loss acquisition, the only peaks detected are the ones with the loss of the specific mass, in this case, 57. Depending on the specificity of the loss, numerous ions can be detected. This leads to complex total ion chromatograms. Therefore, when triggering by a specific mass, the collected target must contain the precursor of interest and have a specific neutral loss.
When using this mode of acquisition and collection, all the peaks with a specific neutral loss are collected. This functionality is valuable when the metabolites have a specific loss related to the drug’s structure. It could also be used for isolating a class of metabolites with a generic loss (e.g., sulfates (–80) or glucuronides (–176)). The precursor mass for each fraction can then be extracted and used to aid in the identification of the metabolites.
In the constant neutral loss example shown, collection could also have been triggered from the total ion chromatogram (TIC). All peaks in the –57 TIC would be collected and then additional analysis or data review would be required to find the desired fractions.
A third mode of fraction triggering is from precursor ion acquisition, as illustrated in Figure 9. Here, MS1 is scanning and MS2 is fixed on a specific product ion. If the specific product ion is observed, it is registered at the detector. The spectrum only shows the masses that have that specific product.
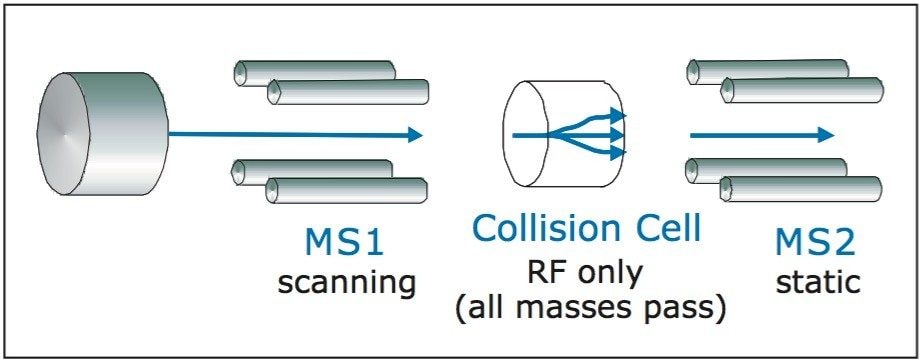
Fraction collection from a precursor ion acquisition has to be from the TIC, since the precursor mass is unknown. This mode of fraction collection is valuable when the metabolites are unknown, but there is a common fragment of the core compound that can be detected.
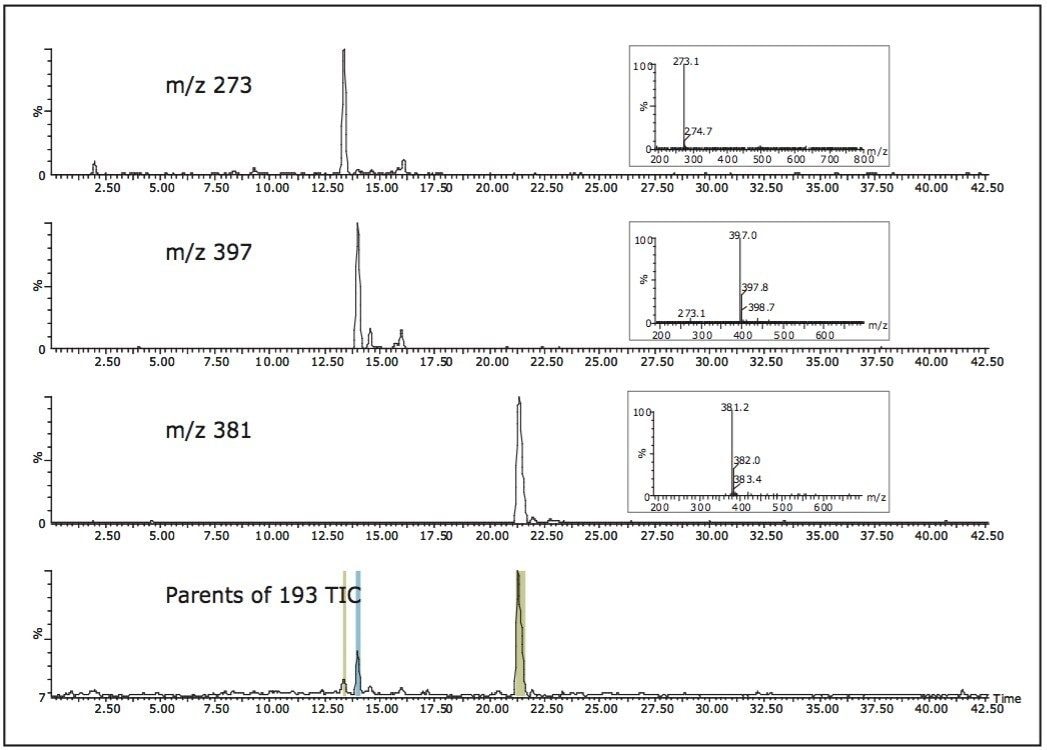
To illustrate the common fragment ion collection capability, Figure 10 shows the glucuronic acid conjugates collected from the ibuprofen urine samples using the precursor ion scan mode of m/z 193. There are three fractions that are collected, m/z 273 (not drug-related), m/z 397 (hydroxyglucuronide conjugate), and m/z 381 (glucuronide conjugate).
The ESCi multi-mode ionization source enables both ESI +/- and APCI +/- acquisition to occur within the same run. This allows for fraction collection to be triggered from any of the acquisition channels, thus proving useful if the metabolites require different ionization modes. Prior to this enabling technology, the only options for collection would be to split the sample and run in different modes, or rely upon time-based fractionation and then analyze all the fractions by both modes to determine the targets.
The selectivity of the ESCi-enabled fraction collection process can be further enhanced by the use of mixed triggers. This approach uses Boolean logic strings to trigger collection from multiple data traces (e.g., collection can occur only when Mass A is present and Mass B is not, or a peak has to be present in two different traces at the same time for fractionation).
Fraction collection with a tandem quadrupole mass spectrometer is now possible using four different modes of data acquisition: scan, MRM, constant neutral loss, and precursor ion, which enables improved versatility for triggering options.
Thus, these different modes of collection add value to a wide variety of applications previously accomplished with more laborious, time consuming, and less specific methodologies.
720001129, June 2007