
This application note will evaluate the suitability of Waters ACQUITY TQ detector (TQD) for tandem quadrupole based analysis of mycotoxins using multiple reaction monitoring (MRM) experiments. Pistachio, almond, and cashew nut matrices will be used for this analysis.
Many agricultural crops are susceptible to colonization by molds and fungi. Stress during plant growth or poor post-harvest storage conditions allow fungal species to infect a variety of commodities, often leading to unacceptable taste, odor or appearance. It is also possible for some fungal infestations to produce toxic secondary metabolites that have the potential to contaminate both animal feed and food intended for human consumption. These secondary metabolites are known generally as mycotoxins1. The Food and Agriculture Organization of the United Nations (FAO) estimates world losses of foodstuffs due to mycotoxins are in the range of 1000 million tons per year2.
Aflatoxins form part of the mycotoxin family and have been associated with acute liver damage, liver cirrhosis, induction of tumors and teratogenic and other genetic effects3.
From a regulatory standpoint, aflatoxins are considered unavoidable contaminants in foods since they cannot be prevented or eliminated by current good agricultural practices2. The four major naturally produced aflatoxins are B1, B2, G1 and G2 (‘B’ and ‘G’ referring to the blue and green fluorescent colors produced by these compounds under UV light on TLC plates).3 This group of compounds is governed by EU legislation “Commission Regulation (EC) No 1881/2006,” which states that the sum of the concentrations of the four aflatoxins in the edible part of nuts may not exceed 4 μg/kg. The concentration of aflatoxin B1 alone may not exceed 2 μg/kg.
Due to the 16x dilution factor in the extraction procedure, the concentration level of detection that the ACQUITY TQD must achieve for the EU legislation is 0.0625 ng/mL or lower. Similar legislation is enforced in Japan3 and the USA4 with limits of 10 μg/kg and 20 μg/kg for the sum of the four aflatoxins applying, respectively.
The aflatoxins, ochratoxin A, the fumonisins, and tricothecenes such as deoxynivalenol are legislated against in many countries. Rapid, sensitive and accurate analysis may be carried out for these compounds using immunoaffinity test kits. Immunoaffinity sample preparation is also appropriate for chromatography based analysis where the maximum sensitivity and selectivity is required5. In addition, a single analytical method able to target a variety of mycotoxin classes in a range of agricultural produce is desirable in order to obtain more comprehensive information on the range of contaminants that are present in human food. Such a multimycotoxin method is appropriate for laboratories testing food for consumption in the European Union, where the range of contaminants legislated against is the most extensive in the world.
The introduction of the ACQUITY TQD detector (Figure 1) allows scientists to perform mycotoxin analysis while harnessing all the benefits that this new instrument brings to the laboratory. The latest IntelliStart Technology in this instrument is designed to reduce the burden of complicated operation, time-intensive troubleshooting, and upkeep. Its small footprint will give any laboratory an advantage as this powerful tool removes the need for larger instrumentation.
This note describes an extended multi-mycotoxin method, for 25 contaminants in 3 nut matrices, which is able to meet the requirements for the analysis of regulated compounds and also includes a range of the other compounds of concern.

The sample extraction method has been previously reported and remains unchanged.1,6,7,8 Mycotoxin extracts in pistachio, almond and cashew nut matrices were provided along with solvent standards of the compounds by the Food and Consumer Product Safety Authority (VWA), Amsterdam, NL. The extracts were analyzed on the ACQUITY TQD.
|
LC system: |
ACQUITY UPLC System |
|
Column: |
ACQUITY UPLC BEH C18 Column 2.1 x 100 mm, 1.7 μm |
|
Column temp.: |
40 ˚C |
|
Flow rate: |
400 μL/minute |
|
Mobile phase A: |
Water + 0.1% formic acid |
|
Mobile phase B: |
Acetonitrile + 0.1% formic acid |
|
Total run time: |
15 minutes |
|
Injection volume: |
50 μL |
|
Time (min) |
%A |
|---|---|
|
0.0 |
90 |
|
3.0 |
90 |
|
10.0 |
30 |
|
10.1 |
10 |
|
12.0 |
10 |
|
12.1 |
90 |
|
MS System: |
Waters ACQUITY TQ detector |
|
Ionization mode: |
ESI positive |
|
Capillary voltage: |
4 kV |
|
Coen voltage: |
Various |
|
Desolvation gas: |
Nitrogen, 800 L/Hr, 450 ˚C |
|
Cone gas: |
Nitrogen, 5 L/Hr |
|
Source temp.: |
120 ˚C |
|
Acquisition: |
Multiple Reaction Monitoring (MRM) |
|
Collision Gas: |
Argon at 3.5 x 10-3 mBar |
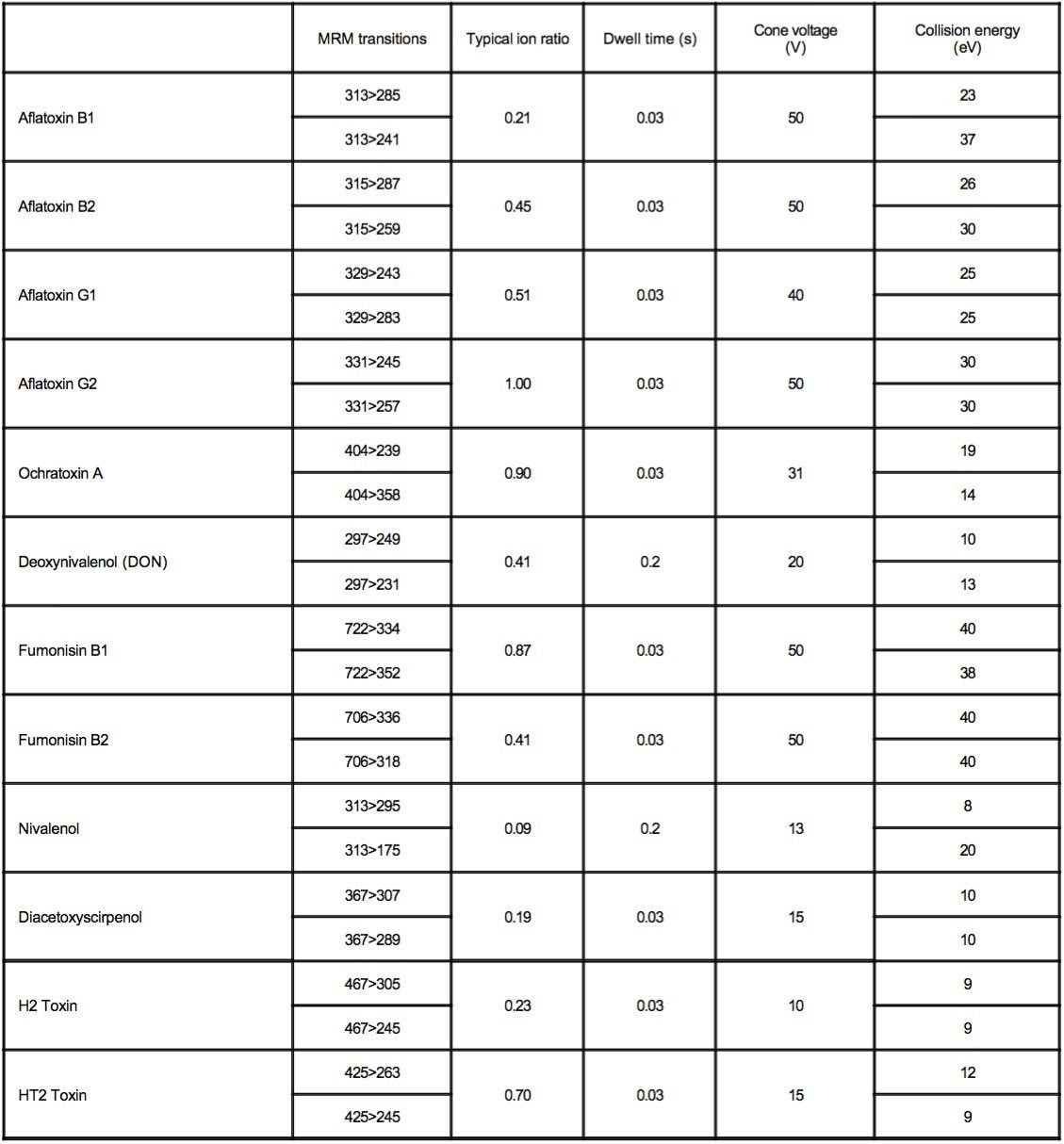
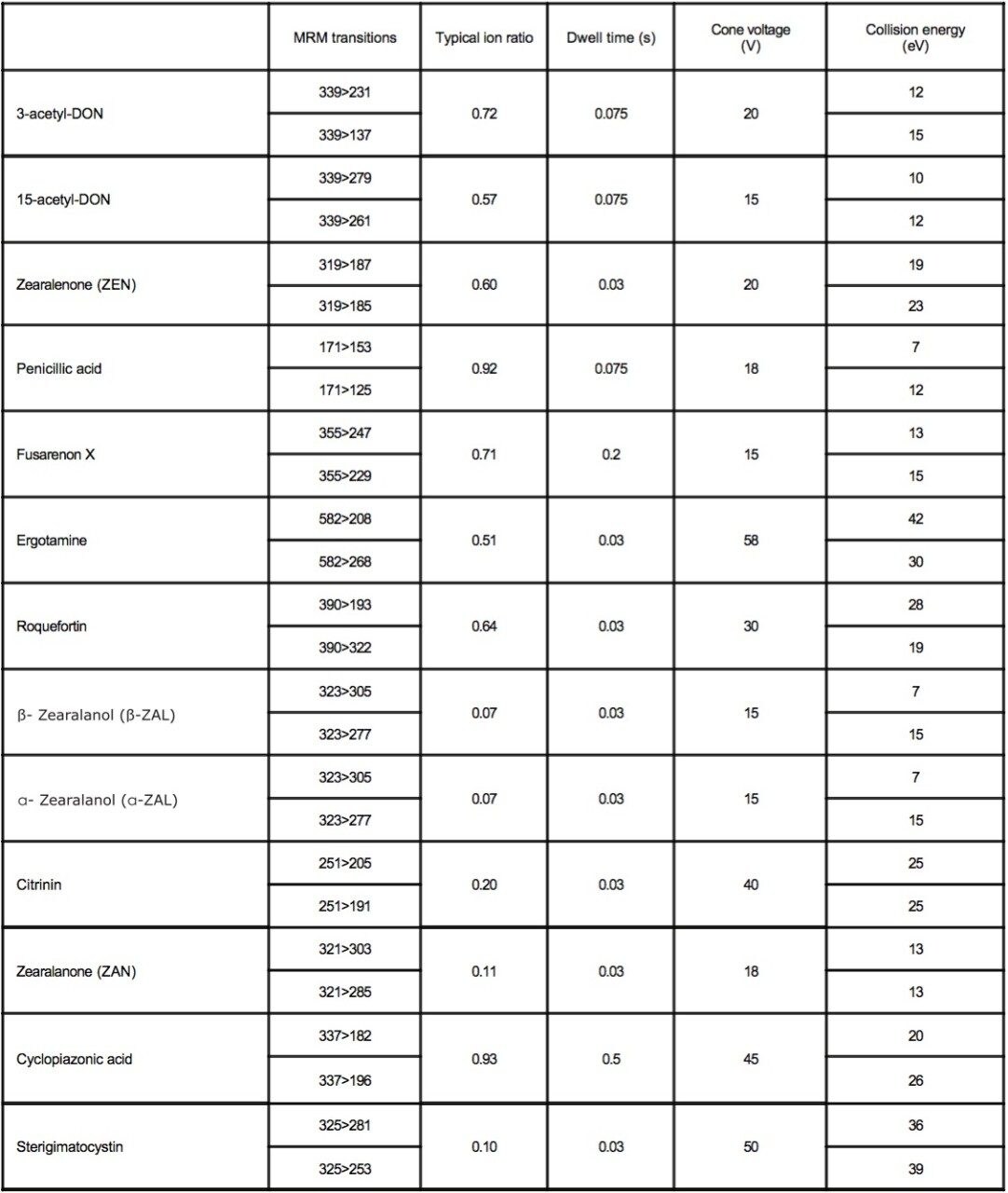
The ACQUITY TQD was tuned so that the precursor and product ions were resolved with a peak width at half height of less than 0.7 Da. The list of mycotoxin residues and the MRM transitions, along with the dwell times, cone voltages, and collision energies for the method are listed in Appendix 1.
Waters MassLynx Software version 4.1 was used for acquisition and its TargetLynx Application Manager was used for data processing.
All 25 compounds were separated successfully. Figure 2 shows the total ion chromatogram for all compounds. Varying dwell times (listed in Appendix 1) and time windows were employed to achieve an average of 12 data points across each peak with Nivalenol eluting first at 1.16 min and Cyclopiazonic acid eluting last at 9.52 minutes.
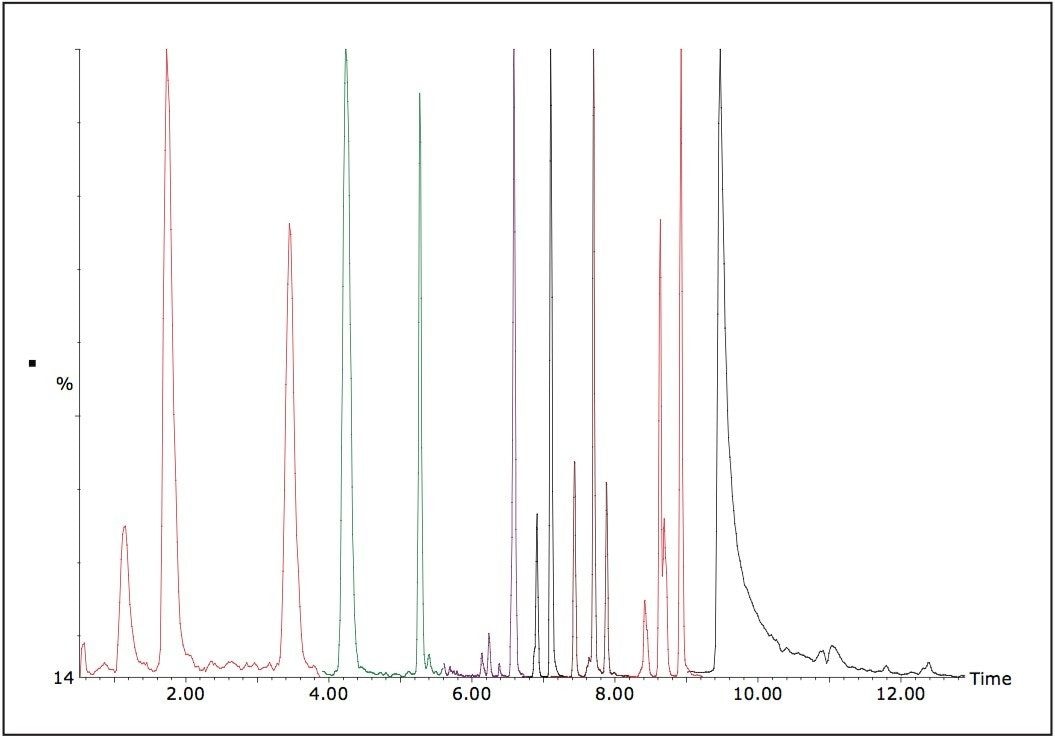
Figure 3 shows the four main aflatoxins in pistachio nut matrix. When lower limits (such as baby foods) have to be achieved, a sample clean up step via SPE9 can be utilized to help reduce matrix effects and concentrate the sample to improve sensitivity.
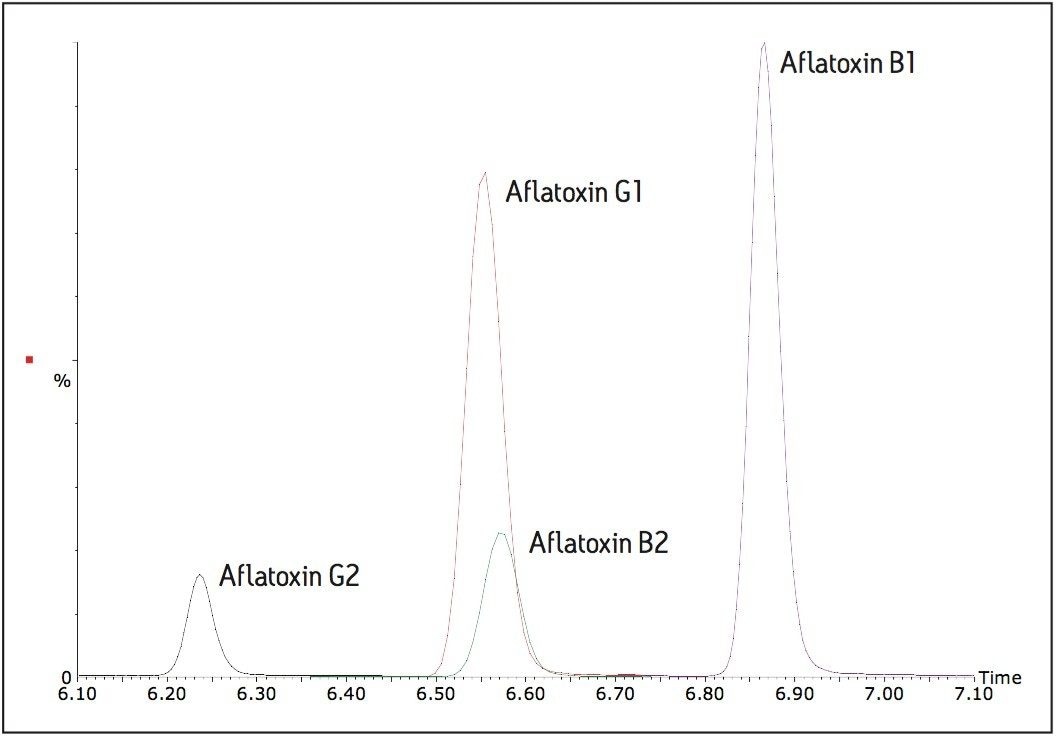
Figure 4 shows the four legislated aflatoxin standards in solvent at 0.0625 ng/mL, corresponding to 1 μg/kg equivalent sample concentration, which is two times below the limit set for aflatoxin B1 in the EU legislation 1881/2006.
The signal to noise ratio (S:N) for the smallest peak, aflatoxin G2 is 5:1 (peak to peak). Thus the TQD can detect all four aflatoxins at concentration levels corresponding with the EU limits.
The aflatoxin solvent standards supplied for this analysis ranged between 0.0625–1.0 ng/mL. Due to the sample extraction method which incorporates a 16x dilution factor, these values are corresponding with an equivalent sample concentration range of 1-16 μg/kg when referring to the edible part of the nut.
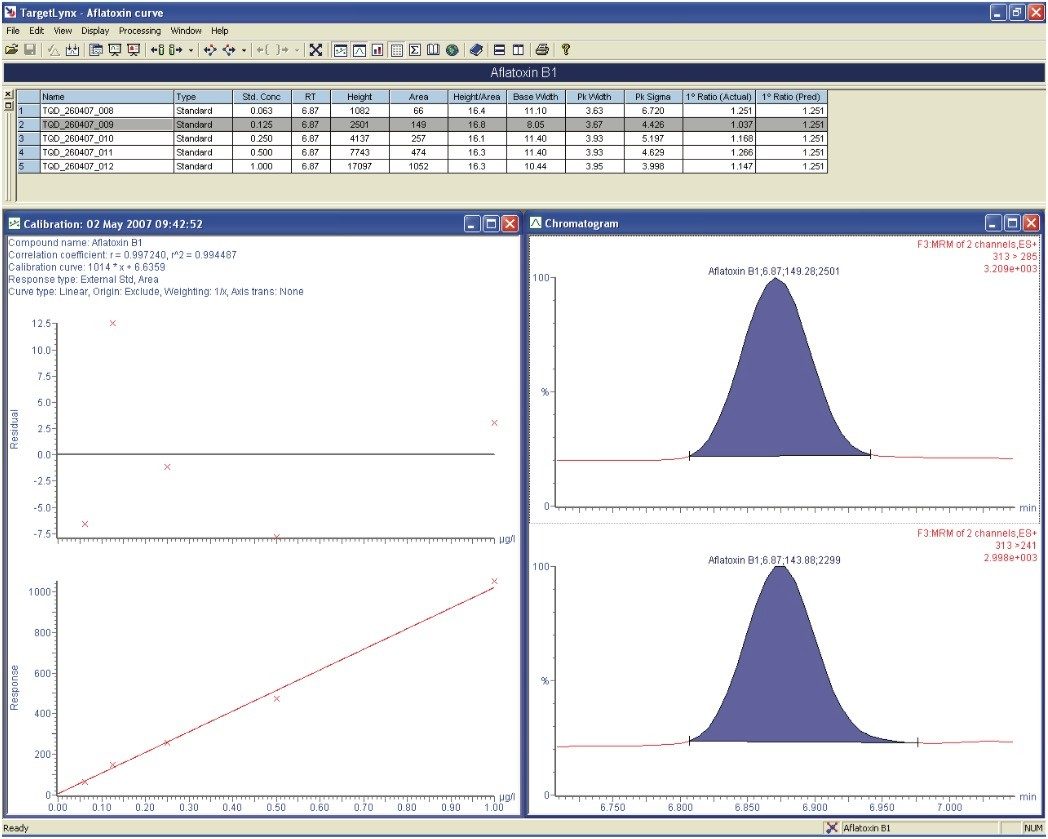
Figure 5, a screenshot of the Targetlynx application manager, shows how linearity was achieved over the concentration range with all standards lying within 15% of their nominal value.
The upper chromatogram, the primary transition, is used for quantification whereas the secondary transition (lower chromatogram) is used for confirmation via the ion ratio. The ion ratio of the sample extract must lie within a 20% tolerance range of the corresponding calibration standard injections’ ion ratio for positive confirmation
Due to limiting sample extract volume, a run of 15 injections in cashew nut matrix was performed to evaluate the robustness of the confirmatory ion ratio. Figure 6 shows the changes in ion ratio over the course of the run. All injections were within the 20% tolerance set with the average difference shown in the header of the graph for each compound.
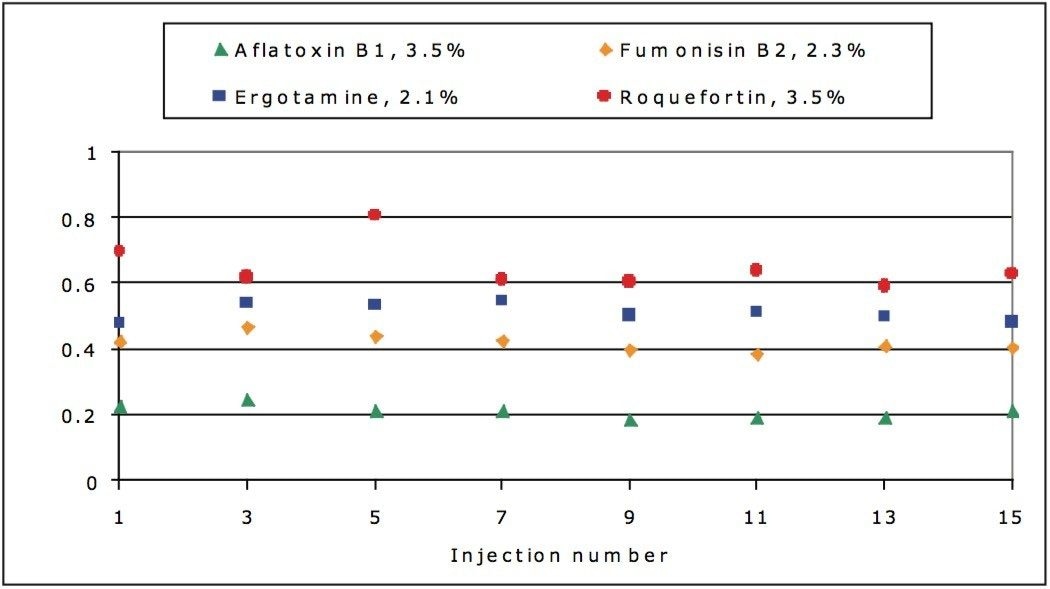
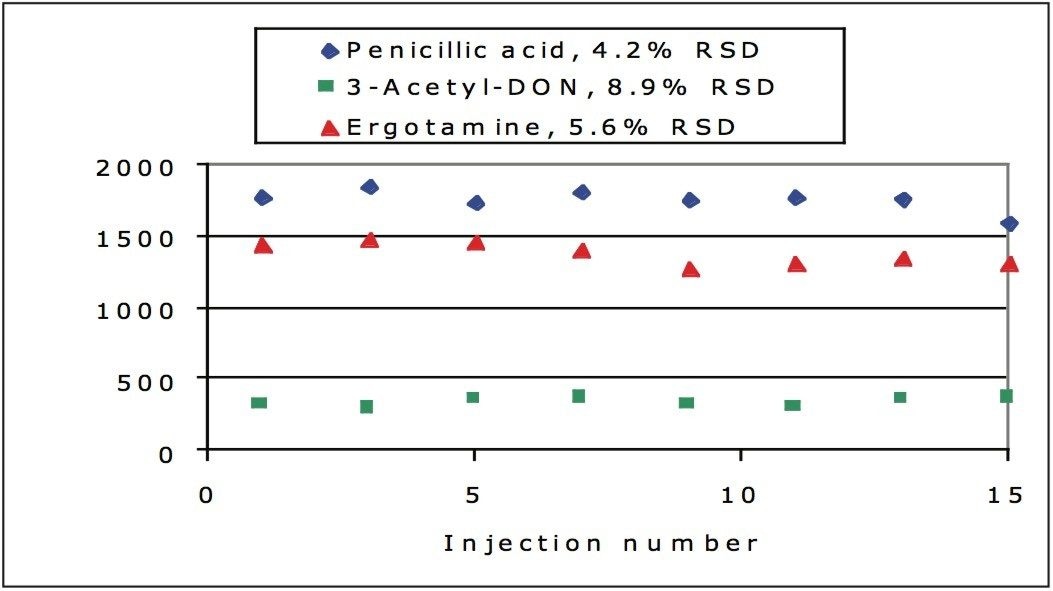
Figure 7 shows the peak area stability over the same 15 injections. The RSD for the compounds is below 9% in all cases.
The contaminants that cause serious harm to human health, such as aflatoxins, ochratoxin A, and trichothecenes, can be quantified using the method described at levels corresponding with the EU legislation limits on ACQUITY TQD.
Ion ratios for confirmation using two MRM transitions were shown to be stable, which is important for quantification and confirmation.
The method is also applicable to the monitoring of various mycotoxin contaminants of emerging concern.
This method allows the determination of multiple contaminants per sample which enables a complete picture to be obtained of exposure to these compounds from the human diet.
This multiple mycotoxin method obsoletes the use of several single mycotoxin methods where repeat analysis is required. The benefits of UPLC for a revenue conscious laboratory are shown with increased speed whilst further reducing solvent usage and therefore the costs of solvents and solvent disposal.
The authors would like to thank the Food and Consumer Product Safety Authority (VWA), Amsterdam, Netherlands, for kindly supplying all the standard solutions, and Elma Bobeldijk in particular, for preparing all the sample extracts that were analyzed in this project.
720002244, July 2007