For research use only. Not for use in diagnostic procedures.
This is an Application Brief and does not contain a detailed Experimental section.
This application brief assess the performance and robustness of HILIC and BEH Amide Column chemistries for the separation of hydrophilic polar metabolites and their suitability for metabolic profiling studies.
The BEH Amide Column demonstrated excellent polar compound retention, chromatographic peak shape, and reproducibility for the analysis of standard mixtures and human urine thus proving to be suitable for large scale metabolomic studies.
Biological matrices typically employed in metabolic profiling studies consist of a wide variety of small molecules pivotal in many biochemical processes. Pathways such as the citric acid cycle and those associated with amino acid metabolism contain very polar compounds that are difficult to retain and resolve by reversed-phase (RP) chromatographic conditions. Hydrophilic interaction liquid chromatography (HILIC) is a technique better suited to the analysis of these polar metabolites which relies on the hydrophilic nature of these compounds to retain them on column. HILIC columns tend to comprise of unbonded silica particles with some stationary phases containing polar functional groups to improve retention and separation of polar analytes that span a wide range of polarity, pKa, and structural moiety. Due to the variability of molecules present in biological matrices and fundamentally their differences in physiochemical properties, the true benefit of bonded versus unbonded stationary phases for profiling experiments is assessed.
To assess the differences in polar retention/separation for unbonded and bonded stationary phases, two HILIC columns were selected for investigation, the Waters BEH HILIC unbonded Silica Column (p/n: 186003461) and BEH Amide Column (p/n: 186004801). In order to assess differences in column characteristics, the Waters LCMS QC Reference Standard Mixture (p/n: 186006963) and a custom mixture of polar endogenous compounds were analyzed under four different mobile phase conditions (Table 1). The custom polar compound mixture contained phenylalanine, taurine, betaine, creatinine, hippuric acid, and benzoic acid. Each standard solution supplied in 100% water was diluted 1:4 with acetonitrile and injected in replicate (n=6). The column temperature and gradient profile was maintained across all column and mobile phase conditions at 40 ºC and 0–100% A for 7 mins respectively. The mobile phase flow rate was also maintained at 0.5 mL/min for all conditions.
All data were acquired using a Waters SYNAPT XS System, operated in resolution mode and data collected in positive ion mode. Extracted ion chromatograms for each of the standards were processed using MassLynx and Skyline (MacCoss Lab, University of Washington, Seattle, USA), where characteristics of chromatographic performance were calculated, including peak widths (Wb), retention factor (K’), selectivity (α), and theoretical plate count (N) in order to determine the optimal column across all the conditions evaluated.
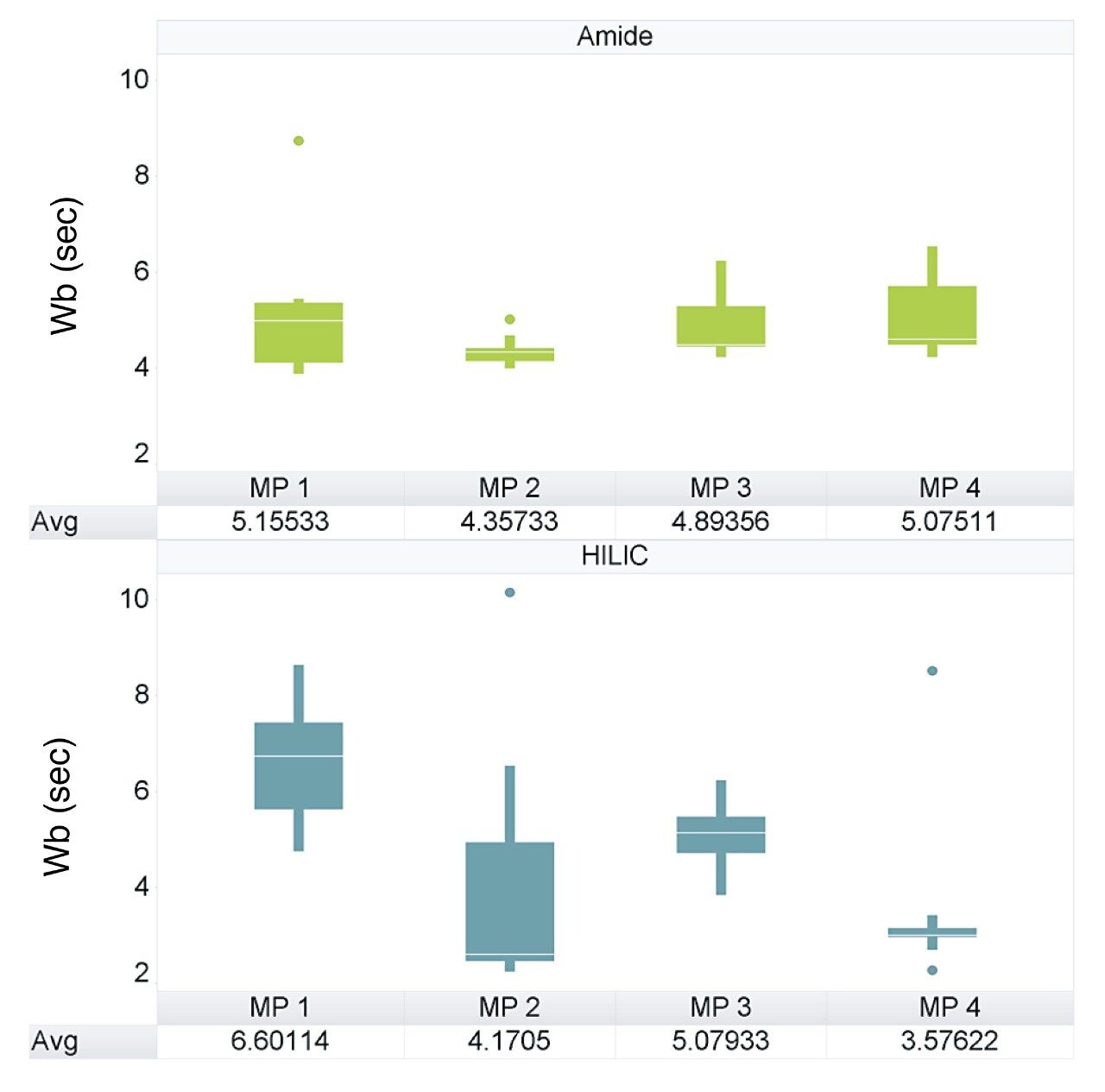
Examining the range of peak widths generated, indicated that the amide column provided much greater consistency, with average widths ranging between 4 and 5 sec across all four mobile phase conditions (Figure 1). The BEH HILIC Column exhibited greater variation in peak widths which was shown to improve with the addition of ammonium acetate (Figure 2), but ultimately ranged from 2 to 9 sec peak widths.
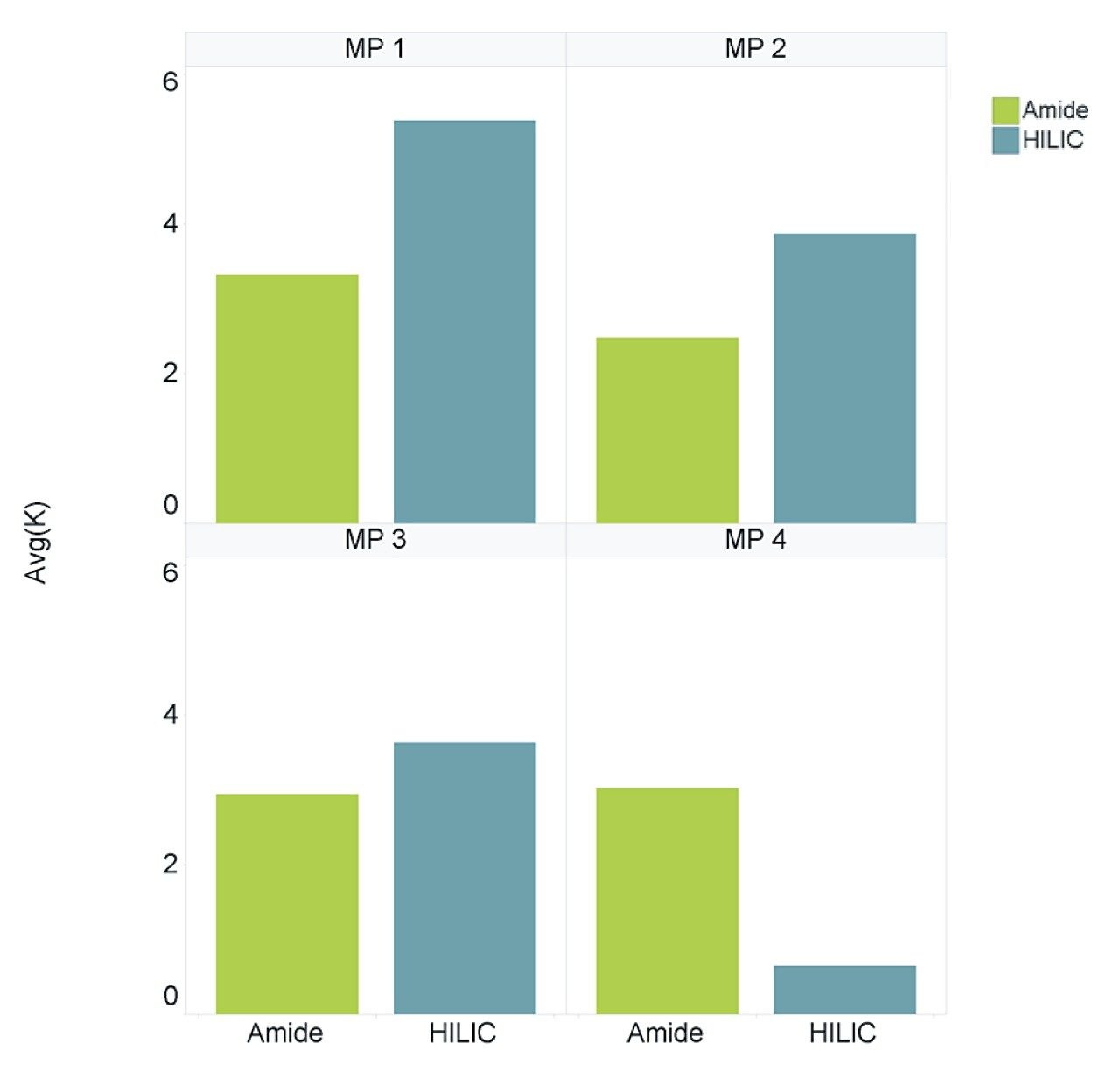
The retention factor (K’) is a measurement of the retention performance and capacity of the column. K’ values between 1 and 10 are generally deemed acceptable during method development and validation.1 Figure 3 shows the average K’ for the LCMS QC reference compounds, separated using both columns under all four mobile phase conditions. Both columns provided an average K’ of >1.5 for the majority of mobile phase conditions assessed, however, the BEH Amide Column is shown to be more consistent over all 4 mobile phase conditions.
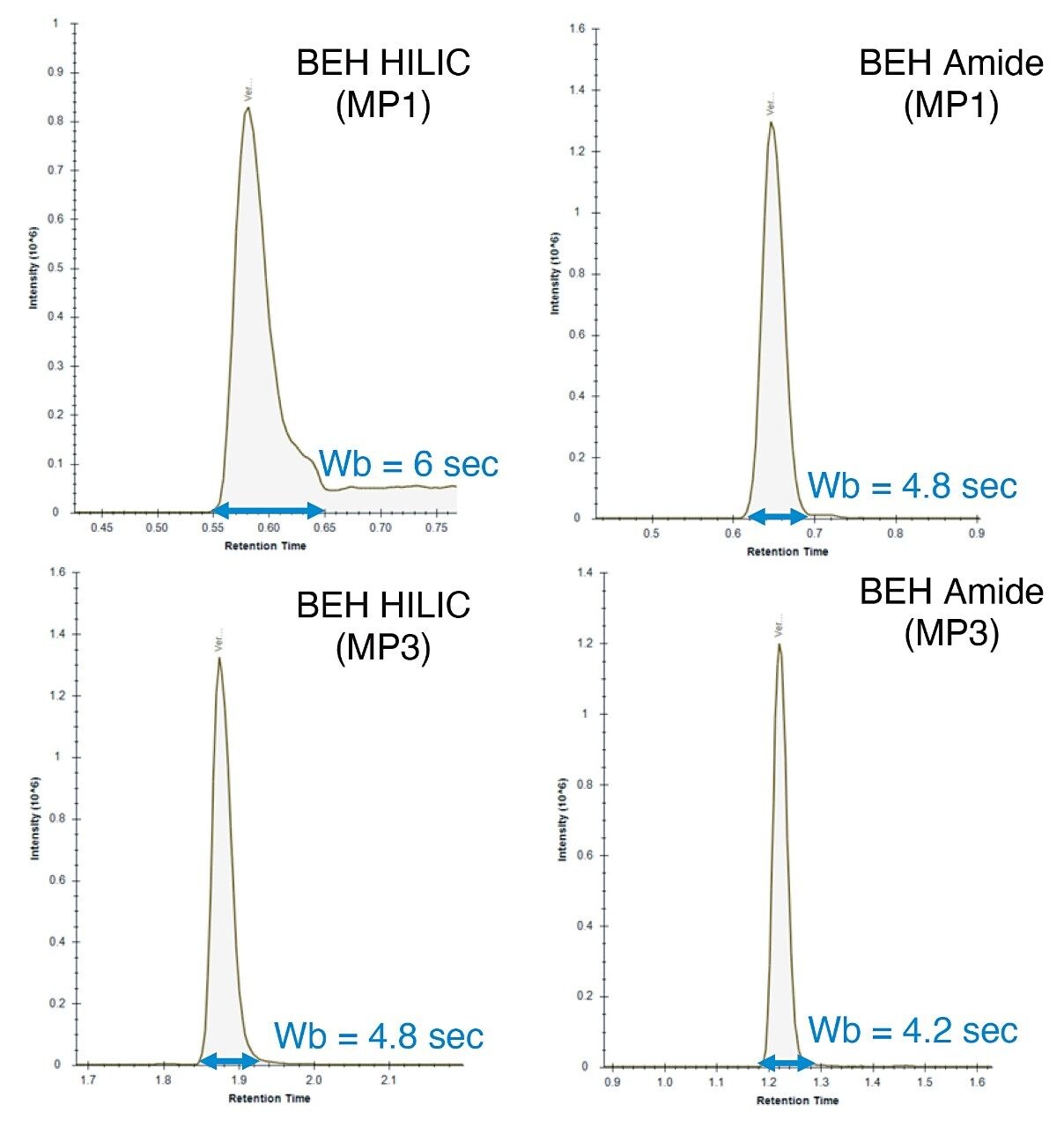
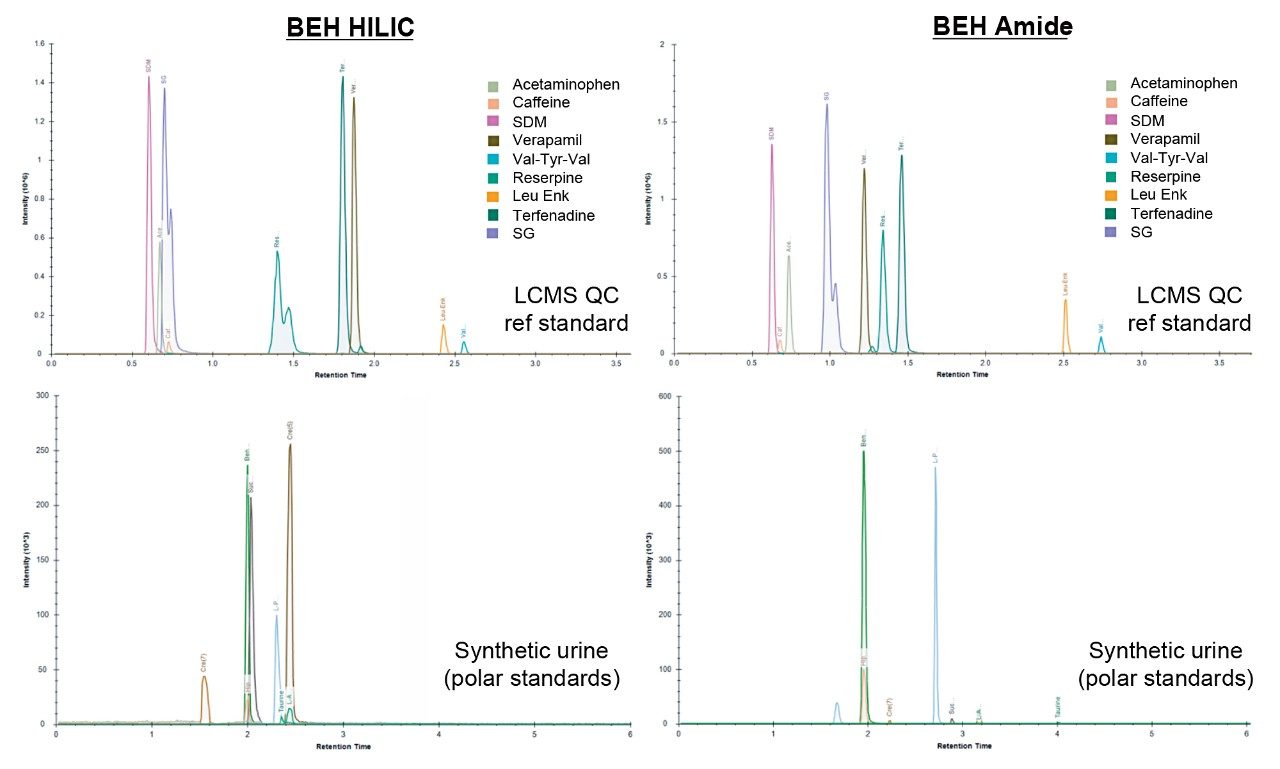
Based on these observations, mobile phase 3 (10 mM ammonium acetate) produced the optimum chromatographic performance for both columns, resulting in similar peak widths and K’ (Figures 1 and 3 respectively). As can be seen in the chromatograms from Figure 4, the BEH Amide Column showed greater separation for the LCMS QC Reference Standard and greater retention for many of the polar standards.
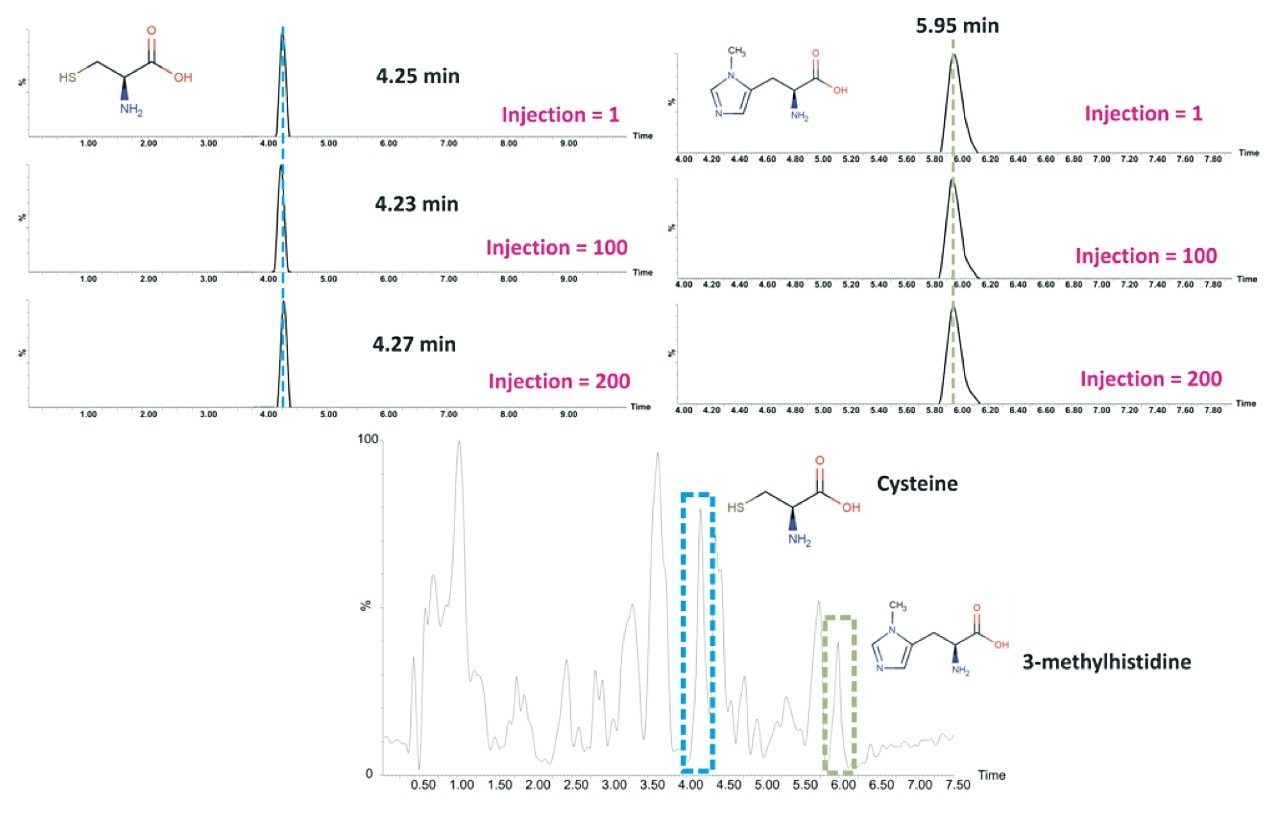
Based on the evaluation outlined, the BEH Amide Column was assessed for reproducibility by acquiring 200 injections (34 hrs of analysis time) of human urine using the optimised chromatographic conditions. The results demonstrated excellent reproducibility over the duration of the analysis (Figure 5).
This investigation highlighted the benefit of bound stationary phase columns for generic metabolic profiling studies when compared with unbound traditional HILIC columns. Improved compound retention, peak shape, and reproducibility for the analysis of polar compounds in complex matrices has been demonstrated. The BEH Amide Column demonstrated improved selectivity and higher peak resolution based on the LCMS QC Reference Standard.
Furthermore, the addition of a buffered mobile phase enhanced the compound-stationary phase interaction leading to sharper peaks and thereby improving chromatographic separation. Assessing the reference standards spiked into human urine showed excellent reproducibility across 200 injections based on the optimized method using the HILIC Amide Column.
720006934, June 2020