
In this application note, we describe a Specific, targeted UPLC-MS/MS method for nitrofurans determination in a range of sample types that meet requirements for both official control and food business operators’ pre-export testing.
The ACQUITY UPLC H-Class System coupled with the Xevo TQ-S micro MS System provides sufficient sensitivity for detection, identification, and quantification of nitrofuran metabolites in a range of products. This method facilitates routine screening and confirmation for official control purposes with a high degree of confidence, but also meets the requirements of pre-export testing, which often demands lower limits of quantification.
Specific, targeted method for nitrofurans determination in a range of sample types that meet requirements for both official control and food business operators’ pre-export testing.
Nitrofurans (NFs) are a group of broad spectrum antibiotics. Due to health concerns, nitrofurans are now prohibited for use in food-producing animals in most jurisdictions. They are still authorized for human medicine and for the treatment of non-food animals. Nitrofurans are widely manufactured, sold, and hence available for misuse.1
There have been frequent findings of nitrofuran residues in honey, poultry, and aquaculture products imported to EU countries, which has led to product recalls, border rejections, and de-listed suppliers. Violations have resulted in implementation of emergency measures that have necessitated mandatory pre-export testing (PET), widespread voluntary pre-harvest tests (PHT), and an increase both in the analysis of imports at border control within the EU and in the frequency of European Commission Food and Veterinary Office (FVO) visits.2
The EU Minimum Required Performance Limit (MRPL) in poultry meat and aquaculture is 1 µg/kg for each of the four nitrofurans, measured as their respective tissue-bound metabolites (EU Commission Decision 2003).3 MRPLs are ‘the minimum content of an analyte in a sample, which at least has to be detected and confirmed’. They are also the reference point for action (Action Levels) when evaluating food consignments. Laboratories must demonstrate that their calculated Detection Capability (CCβ) and Decision Limit (CCα) values are at or below the MRPL.4 Although enforcement action is only taken when a residue exceeds the MRPL, non-compliant samples below the MRPL must still be monitored. Suppliers and importers can set even lower limits for PET based upon trading decisions to provide better warranties to their customers and to gain commercial advantage. Meeting these requirements requires the continued development of highly sensitive and specific analytical methodology based upon UPLC-MS/MS.
Previous studies have demonstrated that parent nitrofurans deplete rapidly in animals and that they are extensively metabolized to tissue-bound metabolites.5 Methods have been described for various animal tissues such as kidney for official control, muscle, honey, shrimp, eggs, and milk for assessing risk to the consumer. Parent nitrofurans are only sought in medicated feeds used for animal production and aquaculture.
Commonly sought parent nitrofurans and associated metabolites include: furazolidone as 3-amino-2-oxazolidinone (AOZ), nitrofurazone as semicarbazide (SCA), furaltadone as 3-amino-5-morpholinomethyl-2-oxazolidinone (AMOZ) and nitrofurantoin as 1-aminohydantoin (AHD).
Derivatization improves extraction efficiency from the acidic aqueous phase into the organic solvent, imparts functionality amenable to reversed-phase chromatography for both SPE and UPLC, and improves electrospray ionization efficiency.
Extracts were generated by Fera Science, Ltd. using a method that was originally developed as part of the “FoodBRAND” Project5 but subsequently modified for routine surveillance. Although newer technology has enabled improvements in performance, the principles behind procedure have changed little. Tails, shells, and coverings were removed from shrimp samples as SCA has been found to be present naturally in the shells. Breadcrumbs were removed from any breaded scampi, fish and chicken products as SCA is formed during the baking of breaded crust. Any ice glaze was excluded as it is no longer considered part of the food. The sample was washed to remove unbound sources of SCA.
Acid hydrolysis was used to cleave the metabolites bound to proteins or other tissues and to hydrolyse the side-chain of any intact parent drug present, as well as to solubilize any residues. Polar nitrofuran metabolites were derivatized with 2-nitrobenzaldehyde. After adjusting the pH, solid-phase extraction (SPE) was carried out on a C18 type cartridge. For the analysis of the kidney samples, extracts were diluted 5-fold. Stable isotope analogues for each of the analogues were added to all samples. Matrix-extracted calibrants were prepared at a Screening Target Concentration (STC) of 0.5 µg/kg, when screening, and at multiple levels, typically over the range 0.25 to 5.0 µg/kg, for any confirmatory analysis. Sample extracts were filtered prior to subsequent analysis by UPLC-MS/MS.
|
UPLC system: |
ACQUITY UPLC H-Class with FTN autosampler |
|
Column: |
ACQUITY UPLC BEH C18 1.7 μm, 2.1 x 100 mm |
|
Mobile phase A: |
0.5 mM ammonium formate (aq.) |
|
Mobile phase B: |
Methanol |
|
Flow rate: |
0.45 mL/min |
|
Injection volume: |
5 μL |
|
Column temp.: |
45 °C |
|
Sample temp.: |
15 °C |
|
Run time: |
11 min |
|
Time (min) |
%A |
%B |
Curve |
|---|---|---|---|
|
0.00 |
80 |
20 |
– |
|
0.20 |
80 |
20 |
6 |
|
7.00 |
25 |
75 |
6 |
|
7.25 |
0 |
100 |
6 |
|
8.25 |
0 |
100 |
6 |
|
8.26 |
80 |
20 |
6 |
|
MS system: |
Xevo TQ-S micro |
|
Source: |
Electrospray |
|
Ionization mode: |
ESI+ |
|
Capillary voltage: |
0.5 kV |
|
Desolvation temp.: |
650 °C |
|
Desolvation gas flow: |
1000 L/hr |
|
Source temp.: |
150 °C |
|
Cone gas flow: |
150 L/hr |
|
Collision gas flow: |
0.15 mL/min |
|
Nebulizer gas pressure: |
7 Bar |
Two MRM transitions were used for each of the nitrofuran metabolites and one for each stable isotope analogue, all determined as the nitrophenyl (NP-) derivatives. Data were acquired using MassLynx MS Software and processed using TargetLynx XS Application Manager. Table 1 summarizes conditions for all MRM transitions including the retention times. The optimum dwell time was set automatically using the autodwell function based upon 4 s wide peaks and 12 data points per peak.
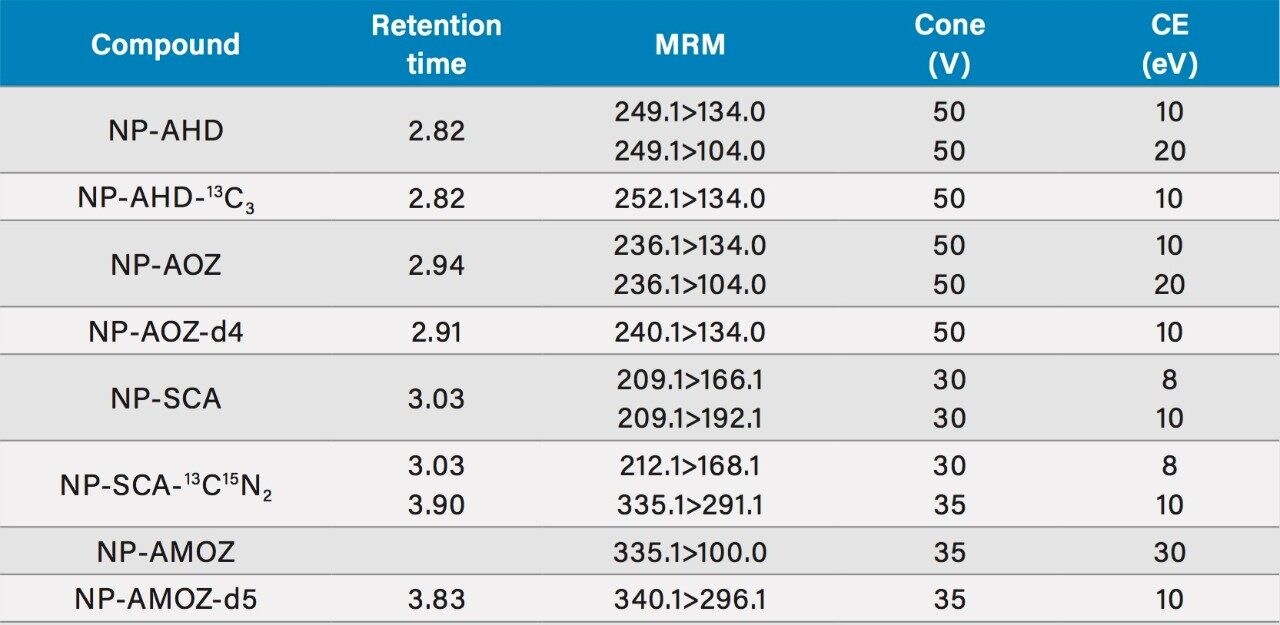
The objective of this work was to evaluate the performance of the Xevo TQ-S micro MS system for nitrofurans analysis rather than development and validation of a new method. Analysis was restricted to batches of different sample types relevant to routine testing. Performance was evaluated based upon sensitivity for the analytes, absence of isobaric interference, precision of the UPLC-MS/MS measurements, compliance with identification and typical quantification criteria, and accuracy and precision through analysis of proficiency test materials.
The UPLC conditions were taken from an earlier Waters application note no. 720002299en,6 but the gradient was adjusted to provide sufficient chromatographic resolution of NP-AHD from isobaric interference on both transitions. The selection of MRM transitions and optimization of critical parameters was performed by infusion of individual solutions of all the analytes and evaluation of the data by IntelliStart Software to automatically create acquisition and processing methods.
Excellent sensitivity and selectivity was demonstrated by the response for each analyte peak detected from the analysis of matrix-extracted calibrants prepared at the STC of 0.5 µg/kg in a range of different sample types: prawn, fish, poultry muscle, bovine kidney, egg, and honey, as shown in Figure 1. No interfering compounds were detected at the retention times of the analytes in all the tested blank samples. Precision, as measured from the peak areas of replicate (n=8) injections of NP-nitrofuran metabolite standards in solvent (1 ng/ml), varied from 1.7 to 3.5 % RSD. Overall repeatability, as determined from the peak areas of the stable isotope analogues added to each of the replicate (n=20) extractions of incurred honey (1 µg/kg), varied from 2.8 to 13 % RSD.
Linearity in matrix (honey) was evaluated over the concentration range 0.2 to 0.5 µg/kg for each of the analytes. The coefficients of determination were satisfactory (r2>0.99), and the residuals <15%. 10 portions of a FAPAS honey proficiency test material contaminated with AMOZ only were prepared and analyzed in duplicate. The mean measured concentration of AMOZ (1.41 µg/kg) compared well with the assigned value (1.50 µg/kg). The precision for the calculated AMOZ concentration in the replicates of the FAPAS test material was 2.9% RSD. Requirements stipulated in EU Commission Decision 2002/65/7/EC for identification were met. The range of calculated AMOZ ion ratios (0.81 to 0.84) was well within tolerance of ±20% (0.66 to 0.99), and the AMOZ retention time from the replicates of the incurred sample showed little variation (0.1% RSD; 3.88 to 3.89 min), well within the acceptance tolerance of ±2.5% (3.79 to 3.98 min).
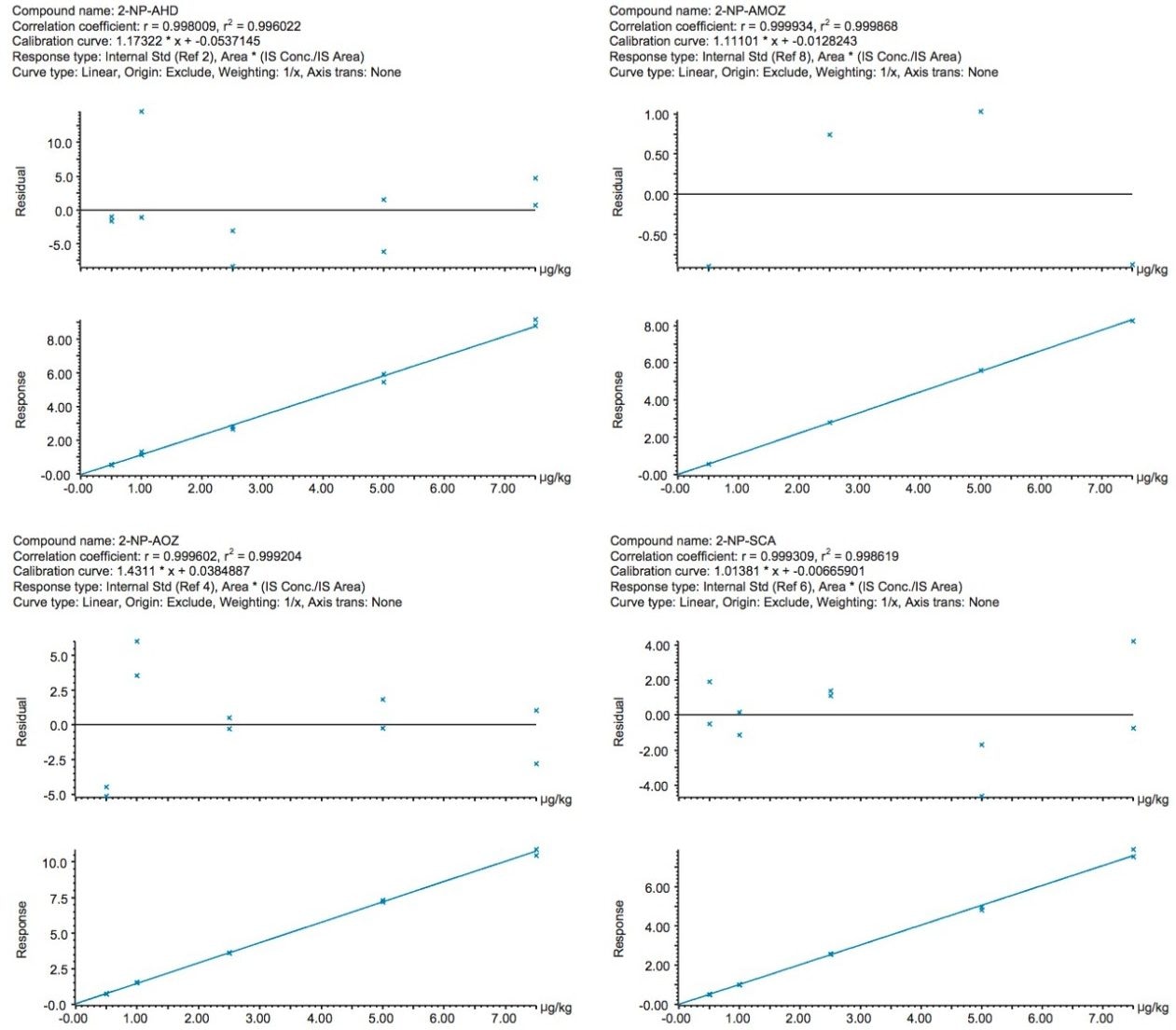
The ACQUITY UPLC H-Class System coupled with the Xevo TQ-S micro MS System provides sufficient sensitivity for detection, identification, and quantification of nitrofuran metabolites in a range of products. This method facilitates routine screening and confirmation for official control purposes with a high degree of confidence, but also meets the requirements of pre-export testing, which often demands lower limits of quantification.
The authors gratefully acknowledge Fera Science Ltd for provision of the extracts for nitrofuran analysis.
720005859, September 2016