The use of a BEH HILIC separation method gives clear advantages over a reversed-phase approach. The increased efficiency in the mass spectrometer’s duty cycle results in increased accuracy of measurement and may contribute to more species being observed. In this experiment, relatively wide tolerances were given to the MRM time window for each class, but greater increases in efficiency may be achievable by using narrower windows.
The translation of lipid separations from HPLC to UPLC have shown marked increases in productivity for lipidomic laboratories. A reversed-phase UPLC method using the C18 HSS T3 column1,2 has distinct and useful features; apart from fast, high resolution separations, it provides clear separation of lysophospholipids and triacylglycerides from other classes of phospholipids. Additionally, the retention time of a species can be predicted with reference to similar species of the same class3. By comparison, a HILIC-UPLC-based method offers the same benefits of speed and sensitivity, but also allows the clear separation of lipids by head group4.
As with any targeted, quantitative metabolomics experiment, maintaining sufficient duty cycle to obtain good quantification is the limiting factor on the number of species that can be analysed simultaneously. Increasing the number of data points across the chromatographic peak is likely to increase the reliability and reproducibility of quantification, as well as possibly increasing limit of detection for the experiment. The HILIC-UPLC method has a potential advantage over reversed-phase methods, as the elution times of lipid classes are predictable, so a reduced list of transitions can be used at a particular time, reducing the duty cycle of the mass spectrometer. To obtain a similar efficiency from a reversedphase method on a tandem quadrupole mass spectrometer would require the building of a comprehensive library of lipid elution times or a complicated elution time model. These considerations of course do not affect time-of-flight (ToF) based mass spectrometers.
Phospholipids were extracted from 100 μL of human serum using the Bligh and Dyer method5, and the dried extract reconstituted in 200 μL of Chloroform:Methanol 1:1 (v/v).
|
LC System: |
ACQUITY UPLC |
|
Column: |
ACQUITY UPLC HSS T3, 2.1 x 100 mm, 1.8 μm |
|
Part Number: |
186003539 |
|
Column Temp: |
65 ˚C |
|
Mobile Phase A: |
40:60 acetonitrile/ water with 10 mM ammonium acetate, pH 5.0 |
|
Mobile Phase B: |
10:90 acetonitrile/ isopropanol with 10 mM ammonium acetate, pH 5.0 |
|
Gradient: |
40–100% B/10 min |
|
Flow Rate: |
500 μL/min |
|
Column: |
ACQUITY UPLC BEH HILIC, 2.1 x 100 mm, 1.7 μm |
|
Part Number: |
186003461 |
|
Column Temp.: |
30 °C |
|
Mobile Phase A: |
95:5 acetonitrile/water with 10 mM ammonium acetate, pH 8.0 |
|
Mobile Phase B: |
50:50 acetonitrile/ water with 10 mM ammonium acetate, pH 8.0 |
|
Gradient: |
0–20% B/10 min |
|
Flow Rate: |
500 μL/min |
|
MS System: |
Xevo TQ-S |
|
Ionization Mode: |
ESI, +/- switching |
|
Capillary Voltage: |
3.0 kV |
|
Desolvation Temp.: |
450 °C |
|
Desolvation Gas: |
1000 L/hr |
|
Source Temp.: |
150 °C |
|
Collision Cell Pressure: |
3.6 x 10-3 mBar |
For HILIC-based separation experiments, the MRM time windows were set across the range of expected elution times for each class. For reversed-phase separation experiments, where elution time is more dependent on aliphatic chain length, the same transitions were studied but the MRMs generally ran throughout the experiment.
The dynamic range of lipid concentrations in plasma provides a challenge for the quantification of all species at a single sample dilution, and hence two dilutions and MRM methods were used (Table 1). For each of the two separation methods, 3 μL was injected 6 times at both undiluted and 100 times dilution in acetonitrile. Peak areas were then extracted automatically using Targetlynx.
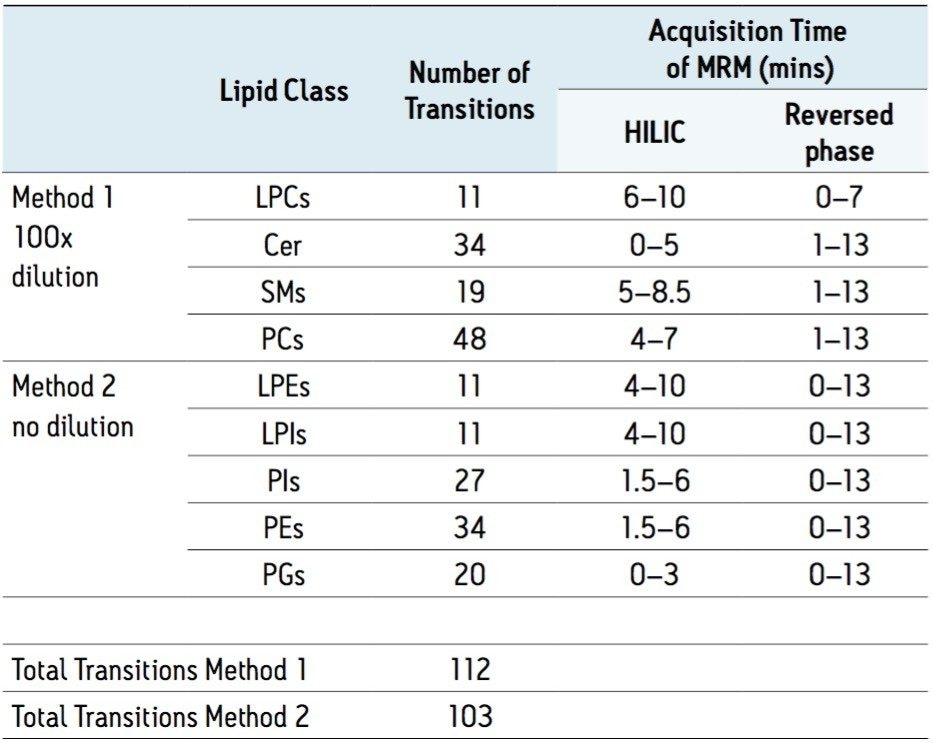
The number of lipids observed reproducibly (6 times out of 6 replicates) above a threshold of 1,000 peak area counts for both UPLC methods are shown in Table 2, below, along with the relative deviations of averaged intensity (%RSD) of each lipid class, Table 1, and of the overall experiment. The %RSDs were calculated based on the peak areas for each lipid.
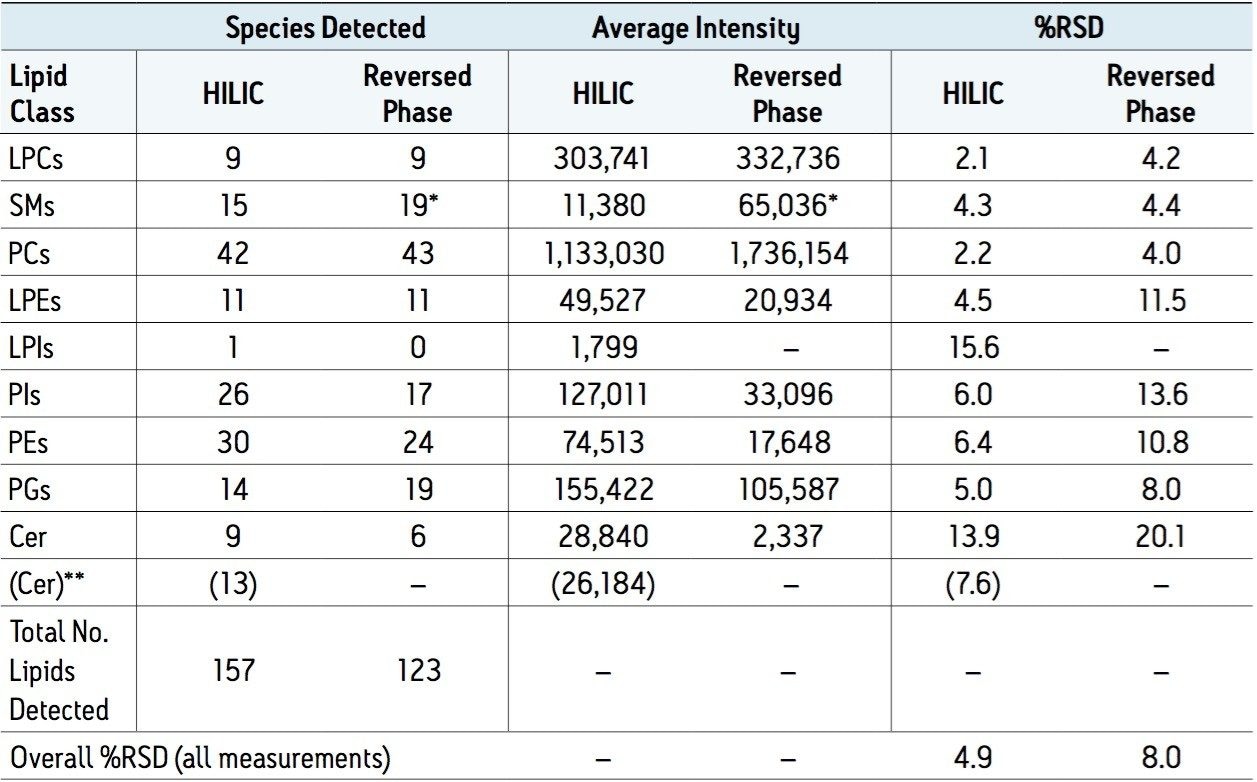
Table 2. Lipid Species Observed by Class.
Numbers of lipid species observed by class, with the average peak area counts and %RSD for each class, as well as overall %RSD per experiment as a whole for six replicate injections by two separation method . *Sphingomyelins detected in the reversed-phase method are difficult to distinguish from isotopic interference from phospotidylcholines, hence assignments may be incorrect. **Ceramides prepared through the Ostro sample preparation route6.
Both experimental methods contain the same number of transitions, however as the HILIC method better synchronises the MRM transitions with elution times of the lipid classes, it is expected that the duty cycle of the HILIC approach will be more efficient. This increase in efficiency is expected to result in better accuracy of quantification, and potentially better limit of detection, manifesting in a greater number of analytes being observed.
The HILIC-based method does indeed detect more lipid species overall than the reversed-phase approach, but this is not true for all of the classes. Each lipid class will have different optimal pHs for ionisation, and as the methods use different mobile phase pHs, it is likely that each method will show better results with some classes than others. How much of this is pH related and how much is due to duty cycle issues is unclear.
Regardless of the number of species identified, and small differences in intensity, the HILIC approach has consistently lower %RSDs for the peak areas than the reversed-phase method, which could be a reflection of the improved duty cycle afforded by the HILIC method.
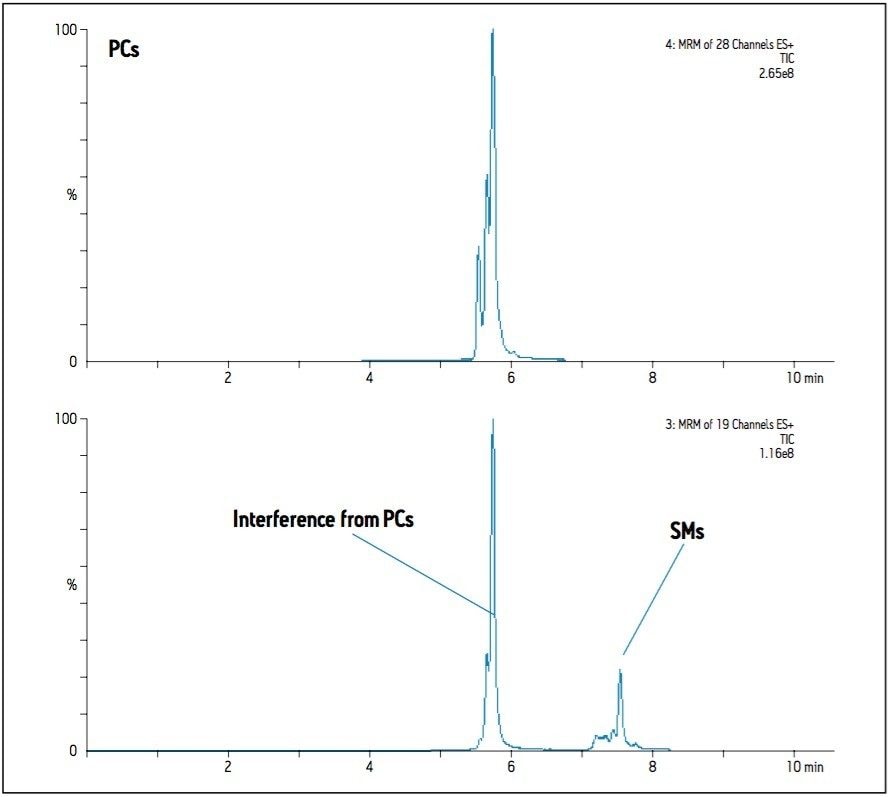
In the case of Sphingomyelins (SMs), the reversed-phase method reports both more species observed and significantly greater intensities when compared to the HILIC method. Precursor ions of the SMs and the PCs of closest mass differ by only one Dalton, and both species share the same diagnostic fragment ion; m/z 184. This results in interference between the transitions of these two classes as the C13 isotopes of one are isobaric with the C12 isotopes of the other. Since PCs are significantly more abundant than SMs in plasma, this affects SM detection and measurement more than it does the PCs unless the two classes can be distinguished. The reversed-phase method provides separation based on aliphatic chain length and number of double bonds, so it is difficult to predict the elution time of a particular class, hence isotopic interferences from PCs are not easily distinguished, as illustrated in Figure 1. Using the HILIC-UPLC method, the two classes are now separated by about 2 minutes (class apex to class apex), and the extent of isotopic interference from PCs in the detection of SMs can be seen in Figure 2. The HILIC-UPLC method therefore provides more accurate and reliable data on the abundance and presence of SMs. Comparing Figures 1 and 2 suggests that the reversed-phase data contains false positives and incorrectly measured abundances due to isotopic interference of PCs.
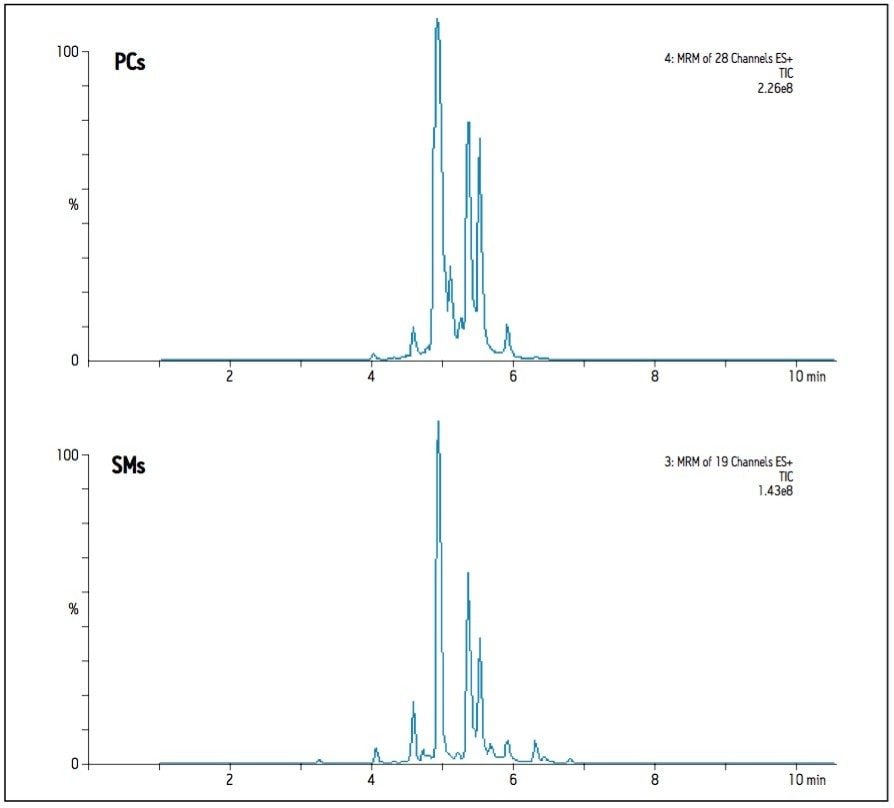
The poorest %RSD with the BEH HILIC method relates to the measurement of ceramides. With this method, the ceramides elute early in the gradient, and may suffer from matrix effects due to un-retained components such as salts. If the same sample is prepared using Ostro sample preparation then more ceramide species are observed, and the intensity measurements are improved.
When taking a targeted approach to lipidomics, the use of a BEH HILIC separation method gives clear advantages over a reversed-phase approach. The increased efficiency in the mass spectrometer’s duty cycle results in increased accuracy of measurement and may contribute to more species being observed. In this experiment, relatively wide tolerances were given to the MRM time window for each class, but greater increases in efficiency may be achievable by using narrower windows (the elution time windows of a particular lipid class are approximately 1 minute wide).
A lipid class based separation approach also gives more reliable information by reducing the likelihood of interference from co-eluting species of a different class. This is most notable with the PCs and SMs.
Many of these considerations benefit only a tandem quadrupole approach. By contrast, the duty cycle of a full scan, high resolution ToF instrument is not affected by a high number of co eluting species and could utilise either a reverse phase or BEH HILIC separation equally well. However, additional information provided by the elution time of the lipid would add confidence to assignments in a class-based separation.
720004219, January 2012