The chromatographic method development process for a bioanalytical LC-MS assay has been simplified by using promotable parameter functions and acquisition templates in the UNIFI Scientific Information System.
Rapidly and efficiently evaluate chromatographic condition screening using promotable parameters and templated data acquisition using the Waters UNIFI Scientific Information System to develop the optimal method conditions.
Selecting the best chromatographic conditions for a bioanalytical assay is often time consuming and requires the careful evaluation of a diverse set of parameters – pH, organic modifier, column chemistry, and gradient steepness, for example – resulting in numerous analytical experiments all of which have to be acquired and reviewed.
The selection of the best methodology is dependent upon obtaining the sharpest peak or greatest response. Consideration must be given to the overall analysis time and resolution of the target analyte(s) from endogenous components in the sample, especially phospholipids that can dramatically reduce analyte response via ion suppression.
Previously, obtaining the most appropriate LC-MS conditions relied upon the knowledge of an experienced bioanalyst and a well-structured experiment.
The Waters UNIFI Scientific Information System facilitates the automated evaluation of multiple analytical variables by the promotion of parameters in the analytical run list. Once developed the analytical run list can be saved as a template for subsequent use.

|
LC system: |
Waters ACQUITY UPLC |
|
LC column: |
ACQUITY UPLC BEH C18 1.7-μm, 2.1 x 50 mm |
|
MS system: |
Waters Xevo TQ-S |
The separations were performed using a mobile phase with either 0.1% formic acid or 0.1% ammonium hydroxide as the aqueous mobile phase and acetonitrile or methanol as the organic modifier.
The mass spectrometer was operated in positive ion mode with MRM data collection, RADAR full scan data collection and precursors of m/z = 184 to monitor the phospholipid elution profile.
Biological samples were prepared by spiking the target analyte into control human plasma. The spiked sample was then precipitated using a 2:1 ratio of acetonitrile to plasma. The resulting sample was centrifuged at 13,000 RPM and the supernatant removed for analysis by LC-MS.
The UNIFI Scientific Information System architecture is scalable to operate in a workstation, workgroup, or enterprise structure. The work for each laboratory or department is maintained in a designated area allowing the templates and methods to be shared within a laboratory team, department or corporation.
This approach allows the implementation of a consistent method development approach via the use of acquisition templates. By taking advantage of the promotable parameter functionality in the UNIFI System, one generic LC analysis method can be selected and then different combinations of aqueous solvent (A) and organic modifier (B) can be chosen for evaluation.
The process of data acquisition is workflow-driven. The user selects to “acquire and process” data, then defines the sample list and analysis method to be used (Figures 2A and 2B).
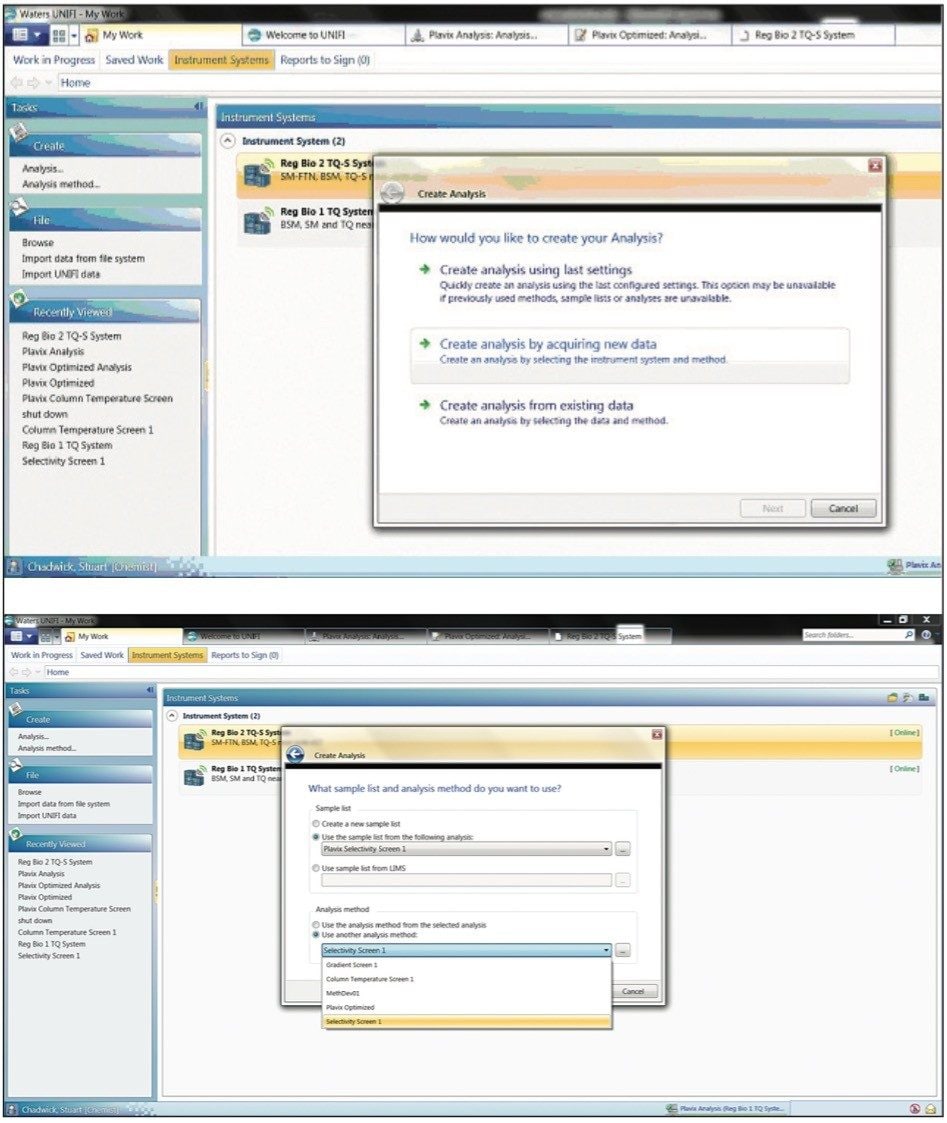
The promotable parameters functionality allows the scientist to select different conditions via a simple drop-down menu. This is illustrated in Figure 3 where different aqueous buffers and organic modifiers are being evaluated.
By using this approach, multiple parameters can be evaluated in one simple, single analytical run. The use of this templated approach removes the need for tedious manual transcription and parameter selection or a series of analytical runs. These templates can be used by all members of the analytical team ensuring a consistent of approach and simple, fast method development.

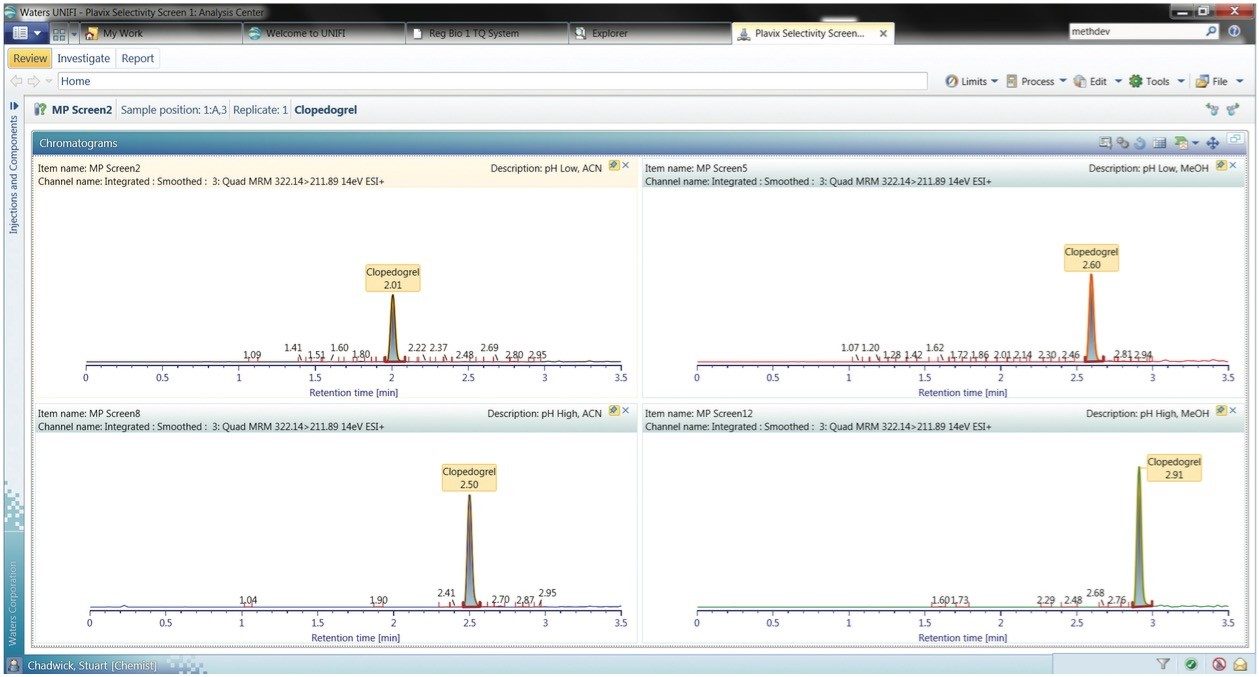
The acquired data can be simply and quickly reviewed. In the example illustrated in Figure 4, the retention characteristics of the anti-platelet compound clopidogrel have been evaluated under acid and basic conditions employing either acetonitrile or methanol as the organic modifier. In this example, we can see that the high pH methanol combination delivers the sharpest chromatographic peak and the greatest analytical response.
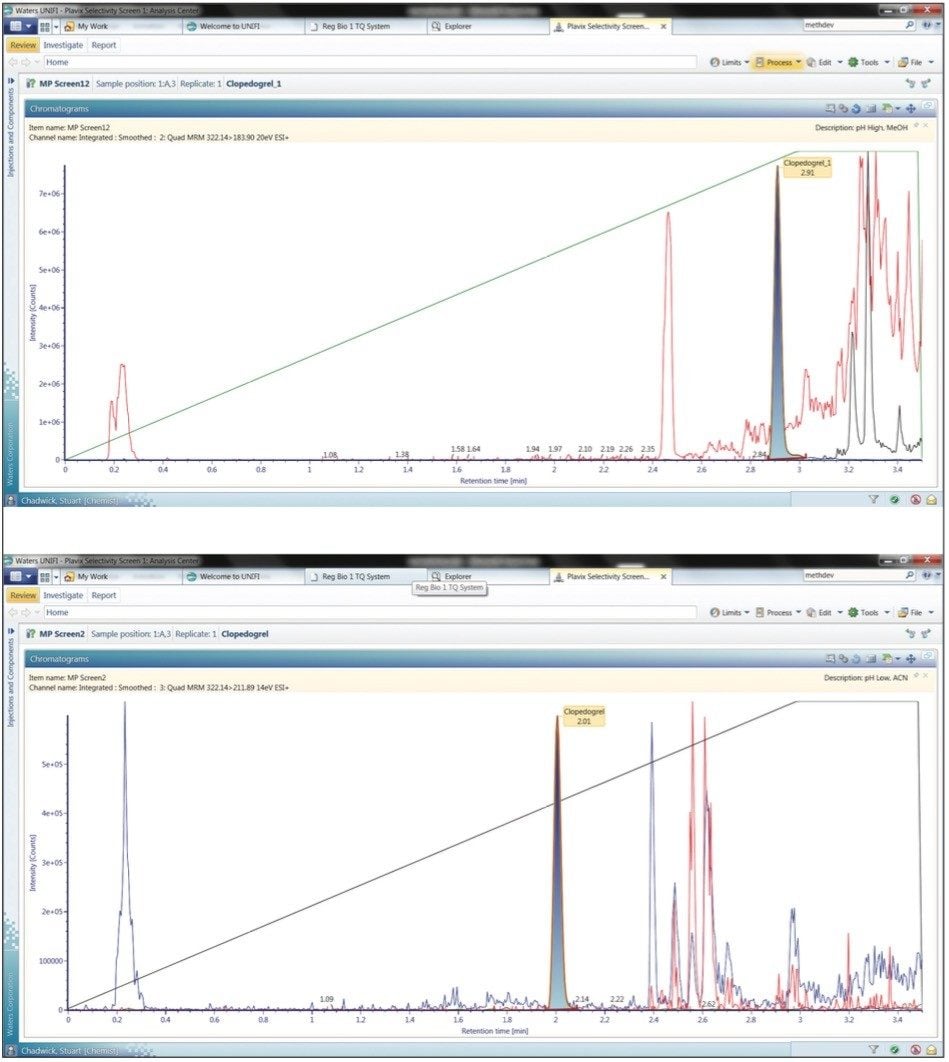
Although the high pH methanol mobile phase gave the greatest response, this is not necessarily the best option of mobile phase combination as the retention characteristics of the matrix components have not been considered.
The simultaneous acquisition of MRM (shaded in blue) and full scan MS data (red) via the RADAR function of the Xevo TQ MS, along with precursors of m/z = 184 (blue) allows the elution profile of the endogenous components of the mobile phase to be evaluated.
We can see from the data displayed in Figure 5A that the use of a high pH aqueous buffer/methanol mobile phase combination causes the analyte to coelute with the lipid fraction of the analyte plasma extract sample. Employing a low pH acetonitrile mobile phase combination results in the separation of the clopidogrel analyte from the endogenous components in the sample such as the phospholipids (Figure 5B).
The chromatographic method development process for a bioanalytical LC-MS assay has been simplified by using promotable parameter functions and acquisition templates in the UNIFI Scientific Information System. The use of this approach:
720004287, March 2012